Published online Mar 14, 2006. doi: 10.3748/wjg.v12.i10.1569
Revised: September 1, 2005
Accepted: October 9, 2005
Published online: March 14, 2006
AIM: To investigate the biological effects of cis-hydroxyproline (CHP) on the rat pancreatic carcinoma cell line DSL6A, and to examine the underlying molecular mechanisms.
METHODS: The effect of CHP on DSL6A cell proliferation was assessed by using BrdU incorporation. The expression of focal adhesion kinase (FAK) was characterized by Western blotting and immunofluorescence. Induction of endoplasmic reticulum (ER) stress was investigated by using RT-PCR and Western blotting for the glucose-related protein-78 (GRP78) and growth arrest and DNA inducible gene (GADD153). Cell viability was determined through measuring the metabolic activity based on the reduction potential of DSL6A cells. Apoptosis was analyzed by detection of caspase-3 activation and cleavage of poly(ADP-ribose) polymerase (PARP) as well as DNA laddering.
RESULTS: In addition to inhibition of proliferation, incubation with CHP induced proteolytic cleavage of FAK and a delocalisation of the enzyme from focal adhesions, followed by a loss of cell adherence. Simultaneously, we could show an increased expression of GRP78 and GADD153, indicating a CHP-mediated activation of the ER stress cascade in the DSL6A cell line. Prolonged incubation of DSL6A cells with CHP finally resulted in apoptotic cell death. Beside L-proline, the inhibition of intracellular proteolysis by addition of a broad spectrum protease inhibitor could abolish the effects of CHP on cellular functions and the molecular processes. In contrast, impeding the activity of apoptosis-executing caspases had no influence on CHP-mediated cell damage.
CONCLUSION: Our data suggest that the initiation of ER stress machinery by CHP leads to an activation of intracellular proteolytic processes, including caspase-independent FAK degradation, resulting in damaging pancreatic carcinoma cells.
- Citation: Mueller C, Emmrich J, Jaster R, Braun D, Liebe S, Sparmann G. Cis-hydroxyproline-induced inhibition of pancreatic cancer cell growth is mediated by endoplasmic reticulum stress. World J Gastroenterol 2006; 12(10): 1569-1576
- URL: https://www.wjgnet.com/1007-9327/full/v12/i10/1569.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i10.1569
The prognosis of pancreatic carcinoma is uniformly fatal. Conventional cytostatic treatments for inoperable pancreatic cancer make little impact on the median survival. Therefore, the development of novel therapeutic concepts that activate proliferation-independent cell death are needed[1]. Cell damage by targeting protein biosynthesis may be a promising strategy for cancer therapy[2,3]. Previous studies have shown that cis-4-hydroxy-L-proline (CHP) as an analogue of L-proline inhibits the collagen synthesis and its extracellular deposition[4,5]. Furthermore, there are reports describing inhibitory effects of CHP on proliferation, adhesion, and migration of various cell types like fibroblasts, epithelial cells and tumor cells[5-8]. These data pointed to additional and/or other proteins whose synthesis might be affected by incorporation of the incorrect amino acid leading to disturbances of cellular mechanisms.
Based on the CHP-induced inhibition of cell adhesion, we hypothesized that the focal adhesion kinase (FAK) may be involved in the CHP-induced signal transduction. FAK is a 125 ku non-receptor tyrosin kinase that mediates different cellular responses to adhesion after clustering of transmembrane integrins at contact sites between the cell and the extracellular matrix (ECM) termed focal adhesions[9]. Frisch et al[10] have shown that constitutively activated FAK conferred protection against anoikis of epithelial cells. Consequently, the inhibition of FAK resulted in apoptosis of fibroblasts as well as in detachment and cell death of human breast tumor cells, suggesting a pivotal role of FAK in the transmission of anti-apoptotic signals[11,12].
The incorporation of amino acid analogues into newly synthesized proteins has been shown to interfere with the correct protein folding leading to an accumulation of misfolded proteins in the endoplasmic reticulum (ER)[13]. The ER has emerged as an organelle that is exquisitely sensitive to alterations in homeostasis that initiates a diversity of molecular defence mechanisms referred to as ‘ER stress’[14]. The complex ER stress response to a variety of different stimuli results eventually in adaptation for survival or induction of apoptosis[14]. Mechanisms activated upon accumulation of unfolded protein in the ER should ensure that only properly folded proteins abandon the ER lumen[15]. This quality control system includes the unfolded protein response (UPR) which is characterized by an up-regulation of chaperons, such as the glucose-regulated protein-78 (GRP)78, as well as by an induction of transcription factors like the pro-apoptotic growth arrest and DNA gene product (GADD)153[16].
The aim of this study was to characterize the biological effects of CHP on the rat pancreatic tumor cell line DSL6A. Addressing the question of the underlying molecular mechanisms, we investigated the participation of ER stress and FAK in the CHP effect on cell proliferation, adhesion, survival and apoptosis.
Dulbecco’s minimal essential medium (DMEM) and fetal calf serum (FCS) were purchased from Biochrom (Berlin, Germany). Iscove’s modified Dulbecco medium (IMDM) was purchased from PAA Laboratories (Cölbe, Germany). Nonessential amino acids, penicillin, streptomycin, trypsin, and all reagents for reverse transcription [Superscript RT, oligo(dT)12-18, dNTP] were purchased from Invitrogen (Karlsruhe, Germany). Cis-4-hydroxy-L-proline were from Riemser Arzneimittel AG (Greifswald, Germany). Colorimetric 5-bromo-2’-deoxyuridine (BrdU)-labelling cell proliferation ELISA and apoptotic DNA ladder kit were obtained from Roche (Mannheim, Germany). CellTiter AQueous kit from Promega (Mannheim, Germany), RNeasy Mini RNA extraction kit and Taq polymerase from Qiagen (Hilden, Germany) were used. Primers for PCR were generated by using NCBI GenBank as the source for any sequences and were synthesized by BioTez (Berlin, Germany). Mouse monoclonal anti-FAK(N354-534) was purchased from Transduction, mouse anti-GRP78 from BD Biosciences, anti-β-actin from Sigma (Taufkirchen, Germany), anti-cleaved caspase-3 from anti-poly-(ADP ribose) polymerase (PARP), anti-cyclinD1 from Santa Cruz. Secondary horseradish peroxidase-labelled anti-mouse or anti-rabbit Ig antibodies, nitrocellulose membranes, ECL plus kit and hyperfilm ECL were purchased from Amersham (Freiburg, Germany). Alexa Fluor® 488 goat anti-mouse IgG was purchased from MoBiTec (Göttingen, Germany). Caspase-3 inhibitor Z-DEVD-FMK, general caspase inhibitor Z-VAD-FMK and the protease arrest reagent were purchased from Calbiochem (Schwalbach, Germany).
The rat pancreas carcinoma cell line DSL6A was cultured in DMEM supplemented with 100 mL/L FCS, 100 U/mL penicillin and 100 µg/mL streptomycin or in IMDM containing non-essential amino acids in addition to 100 mL/L FCS and antibiotics.
In contrast to IMDM, DMEM did not contain L-proline. To prevent cell adhesion, culture plates were coated with 20 g/L agarose as previously described[17].
Cell proliferation was assessed using the colorimetric 5-bromo-2´-deoxyuridine (BrdU) DNA incorporation assay. Cells suspended in DMEM supplemented with 100 mL/L FCS were seeded into 96-well plates (1x104 cells/well). After becoming adherent, the cells were treated for 24 h and 48 h with different CHP concentrations dissolved in serum-free DMEM. BrdU labelling was performed during a further 20-h incubation. The BrdU incorporation was quantified using the ELISA kit according to the manufacturer’s instructions[18].
For the detection of alive cells, the metabolic activity was measured by a colorimetric method based on the conversion of the tetrazolium salt MTS into formazan using the CellTiter AQueous kit according to the manufacturer’s instructions. Cell cultivation and incubation was performed as described for BrdU labelling.
Apoptosis was detected by the cleaved caspase-3 as well as the degradation of poly(ADP-ribose) polymerase (PARP) using the immunoblot technique. To show DNA laddering, cells were seeded into 12-well plates. Adherent cells were cultured in serum-free DMEM for 24 h and 48 h with or without 10 mmol/L CHP. At the end of the incubation, cells were harvested and DNA was extracted using the apoptotic DNA-Ladder kit according to the manufacturer’s instructions. Isolated DNA was electrophoretically separated on 15 g/L agarose gel containing ethidium bromide. The ethidium bromide fluorescence was visualised using an electronic camera.
Total RNA was isolated using the RNeasy extraction kit. Three microgram of total RNA was reversed transcribed into cDNA as previously described[19]. Quantification of mRNA expression was preformed by using competitive PCR for the housekeeping gene β-actin and co-amplification with a defined concentration of a synthetic DNA fragment as internal standard[19]. On this basis, various cDNA samples were adjusted to equal input concentrations and electrophoretically separated on 18 g/L agarose gel containing ethidium bromide. Visualisation and measurement of resulting bands were performed with an electronic camera analysing the data with the EASY program (Herolab, Wiesloch, Germany). The primers for RT-PCR were as follows: for GRP78, GACATTTGCCCCAGAAGAAA (sense) and ATGACCCGCTGATCAAAGTC (antisense), product size 375 bp; for GADD153, AGCTGAGTCTCTGCCTTTCG (sense) and TGTGGTCTC TACCTCCCTGG (antisense), product size 456 bp[20].
Cells were seeded into 60-mm dishes and grown to confluence. Protein extracts of DSL6A cells were prepared as previously described[18]. Proteins were electrophoretically separated on an 80 g/L SDS-polyacrylamide gel and blotted onto nitrocellulose membrane. Blots were incubated with the respective primary antibody for 2 h at room temperature. For visualization of the antibody binding, filters were exposed to a horseradish peroxidase (POD)-labelled anti-rabbit or anti-mouse Ig antibody and developed using the ECL Plus kit. The following primary antibody dilutions were used: FAK 1:1000, GRP78 1:500, cleaved caspase-3 1:500, PARP 1:1000, β-actin 1:1000. For re-probing with additional antibodies, membranes were stripped of bound antibodies by treatment with stripping buffer containing 62.5 mmol/L Tris (pH 6.7), 2 g/L SDS, 7 mL/L 2-mercaptoethanol for 30 min at 50 °C.
Cells were cultivated on glass cover slips as previously described[21]. For immunofluorescence staining, cells were fixed with ice-cold methanol, followed by incubation with a mouse monoclonal antibody (mAB) detecting FAK. Binding of the specific mAB was determined by Alexa Fluor anti-mouse IgG and visualized using the Confocal LASER scanning microscope (Leica TCS SP2 AOBS).
Results were expressed as mean ± SE. Data were analyzed using Wilcoxon’s rank sum test. P < 0.05 was considered statistically significant.
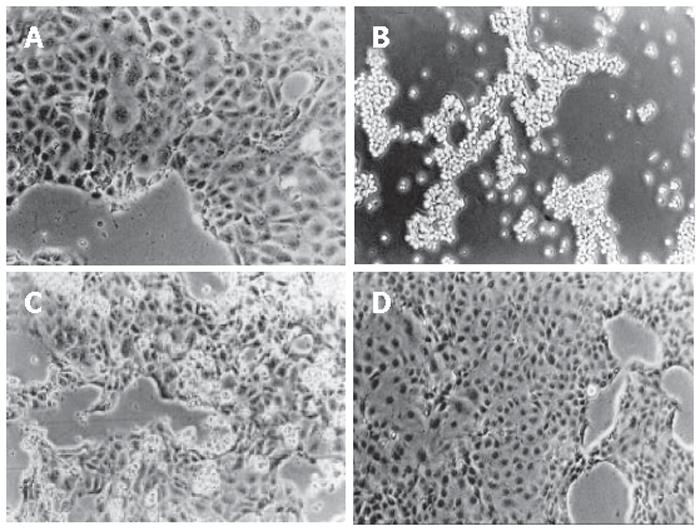
The incubation with 10 mmol/L CHP induced rounding up of adherently growing DSL6A cells resulting after 24 h in a complete detachment (Figure 1B). The CHP antagonist L-proline circumvented this CHP-induced loss of adherence (Figure 1C). Moreover, cells which had been rounded up through CHP treatment, spread out again after L-proline addition, forming finally a normal monolayer (Figure 1D).
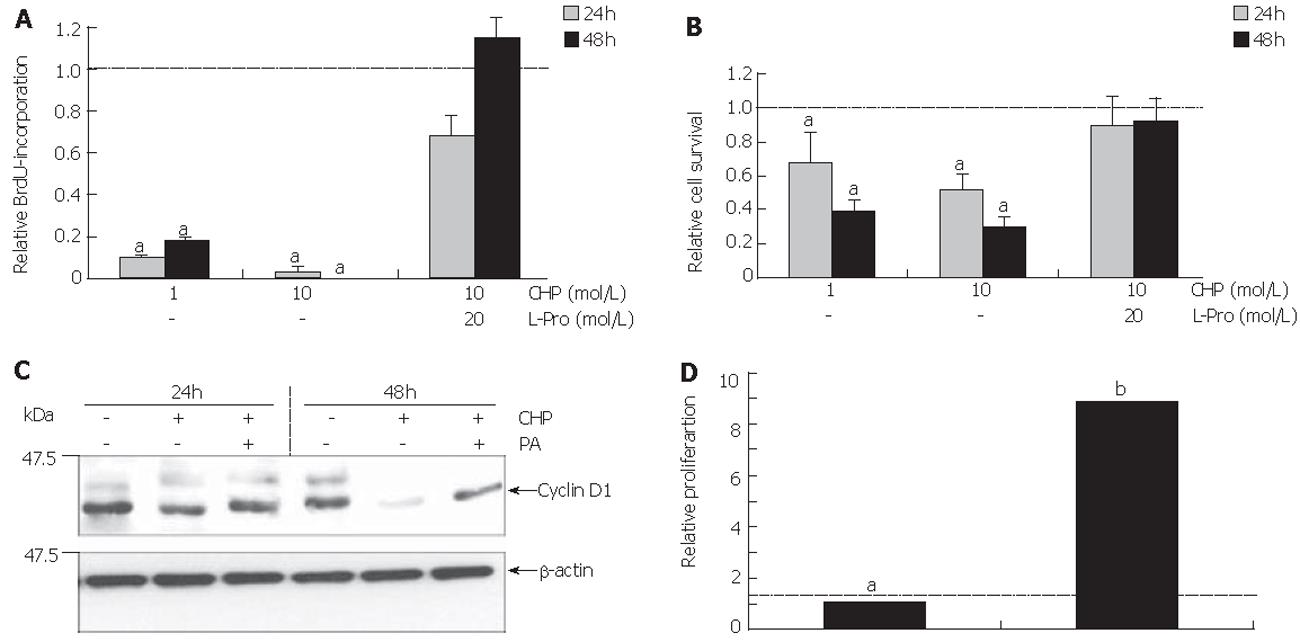
Figure 2A shows the influence of CHP on the S phase of the cell cycle as measured by BrdU incorporation into the DNA of DSL6A cells. We observed CHP significantly inhibited the proliferation in a time- and dose-dependent manner. The determination of metabolically active DSL6A cells (Figure 2B) revealed a discrepancy between the efficiency of CHP in regard to cell proliferation and viability. While the BrdU incorporation after treatment with 10 mmol/L CHP for 24 h averaged only 20% of the controls (Figure 2A), cell viability was more than 50% (Figure 2B). The results obtained by BrdU incorporation have been reflected on the molecular level analysing cyclin D1, a molecule substantially involved in the cell cycle progression[22]. As shown by immunoblotting, CHP triggered a decline of the cyclin D1 protein (Figure 2C).
The CHP-caused growth inhibition could be prevented through addition of L-proline, indicating a competitive replacement of CHP by the correct amino acid (Figures 2A and 2B).
To examine whether the impaired cell functions could be restored, L-proline was added to the CHP-containing culture medium 24 h after beginning of the incubation with CHP. Cells were allowed to grow for further 24 h, followed by the measurement of BrdU incorporation. As shown in Figure 2D, the addition of L-proline induced a re-increase in BrdU incorporation.
In order to exclude the possibility that the restoring of proliferation was due to residual intact cells, we measured the BrdU uptake of cells remaining in the culture plates after removal of cells detached by CHP treatment. The BrdU incorporation was not significantly higher than immediately after CHP incubation (data not shown). These results suggested that CHP-induced DSL6A cell detachment is a reversible process.
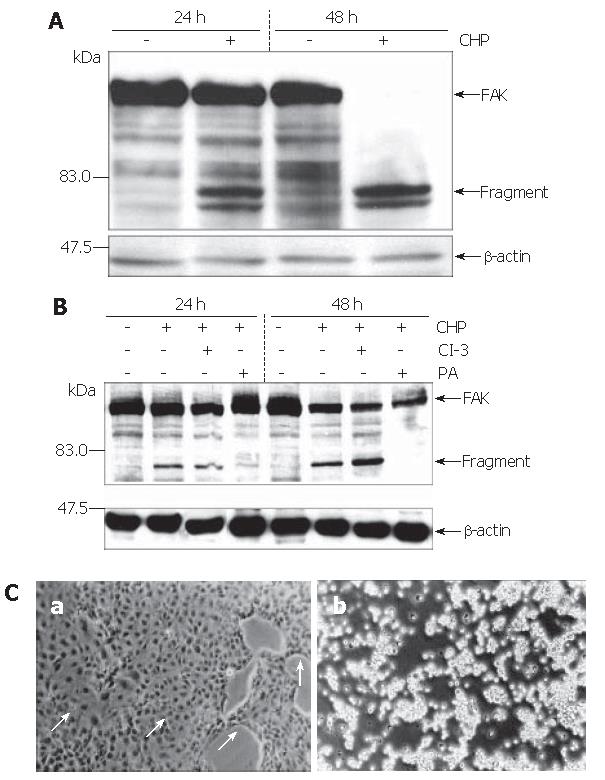
The strong inhibition of cell adherence by CHP pointed to an involvement of the focal adhesion kinase (FAK) in the CHP-mediated molecular mechanisms[10]. Analysis of FAK using the Western blot technique revealed the expected 125 ku protein (Figure 3A). Treatment of the cells with CHP caused a time-dependent decline of the FAK signal and a simultaneous appearance of fragments of about 80 ku, suggesting a proteolytic degradation of the FAK molecule leading to a complete fragmentation during 48 h (Figure 3A).
Based on the hypothesis that CHP brought about FAK inactivation by proteolytic cleavage of the enzyme, we tested the influence of a broad spectrum protease inhibitor [protease arrest (PA) reagent]. The repression of the intracellular proteolytic activity was able to abolish the CHP-induced FAK degradation (Figure 3B). In addition, pre-incubation of DSL6A cell cultures with the protease inhibitor prevented the CHP-caused cell detachment (Figure 3Ca). Moreover, the cyclin D reduction in DSL6A cells treated for 48 h with CHP was attenuated by the PA reagent (Figure 2C)
In contrast, the inhibition of caspase-3 activity by Z-DEVD-FMK (CI-3) had neither an effect on CHP-mediated FAK degradation (Figure 3B) nor on cell detachment (Figure 3Cb). In addition, treatment with Z-VAD-FMK, a general caspase inhibitor including caspase-3, 7, 8, 10, could not abolish CHP-caused influences on DSL6A cells (data not shown).
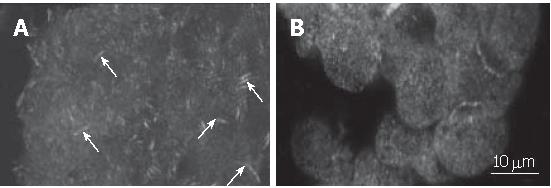
Since FAK is localized in focal adhesions while the cell is in contact with extracellular matrix, we, using immunofluorescence staining, analysed the influence of CHP on the intracellular assembly of the enzyme. Results were visualized by laser scanning microscopy (Figure 4). We clearly observed the focal adhesions in adherently growing control cells (Figure 4A), whereas the CHP-caused cell rounding resulted in a loss of focal adhesions and a diffuse distribution of the FAK protein (Figure 4B).
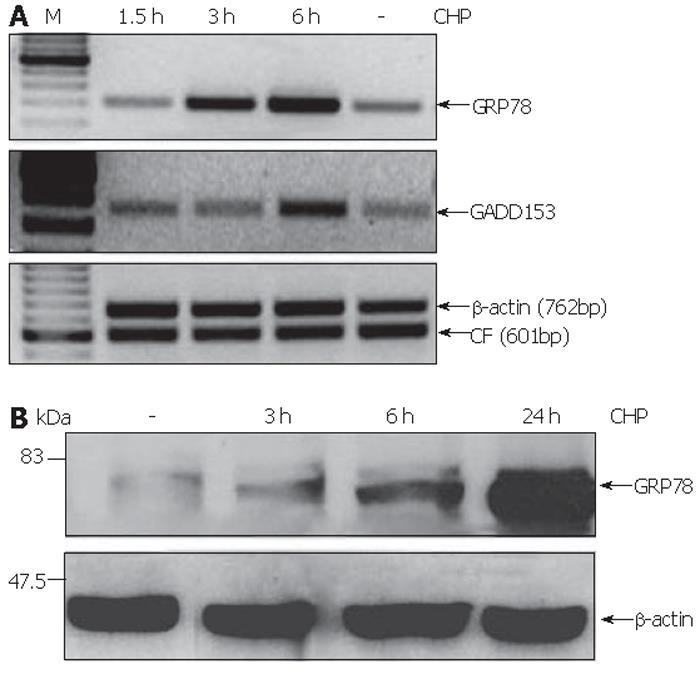
CHP was earlier described as an inhibitor of normal collagen folding[22], a condition that inevitably affects the homeostasis of the endoplasmic reticulum (ER) referred to as ER stress[15]. In order to test whether CHP effects are mediated by ER stress, we analyzed the expression of GRP78 and GADD153 proteins whose up-regulation was defined as a component of the unfolded protein response (UPR)[18,15]. We found that CHP induced the mRNA expression of the both ER stress markers (Figure 5A). GRP78 transcript levels were found to be increased 3 h after the addition of CHP, while the up-regulation of GADD153 was detected only after 6 h. The CHP-induced GRP78 expression was confirmed on protein level by immunoblotting. A modest increase of the UPR component could be detected 3 h after the addition of CHP. The GRP78 protein levels in the cells increased during prolonged incubation with CHP reaching a maximum after 24 h (Figure 5B).
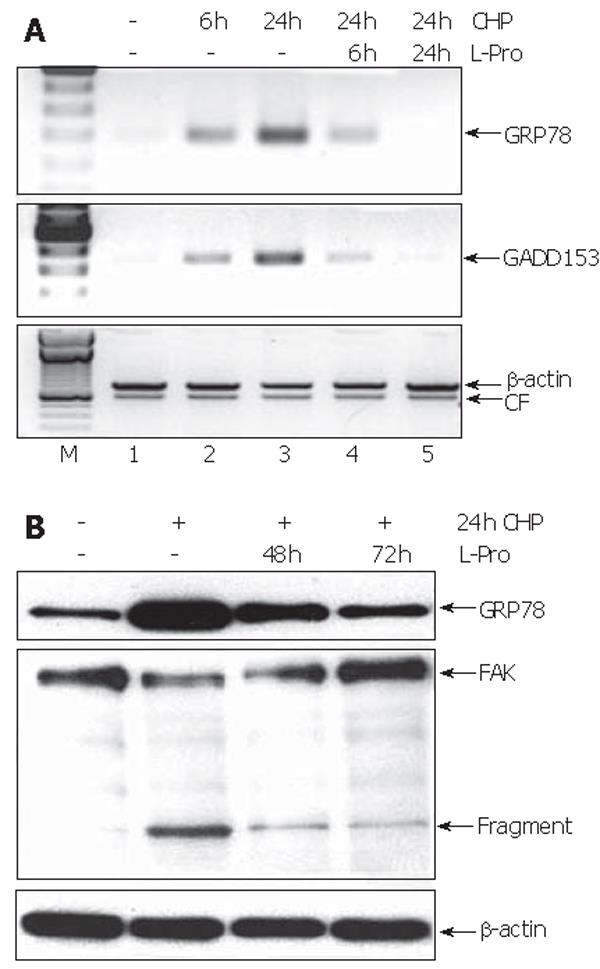
To test whether the L-proline-caused restoration of cell functions (Figure 2) was reflected on the molecular level, we investigated the time course of the expression of GRP78 and GADD157 during incubation with L-proline in CHP-treated cells. Six hours after the addition of L-proline, the transcript levels of both UPR components were reduced, reaching normal values during 24 h (Figure 6A). In contrast, the decrease of GRP78 protein levels could be detected only after 48 h (Figure 6B). Furthermore, L-proline-induced reversion of the CHP-mediated impairment of cell functions was accompanied by a time-dependent reconstitution of the FAK molecule associated with a reduction of the protein fragments (Figure 6B).
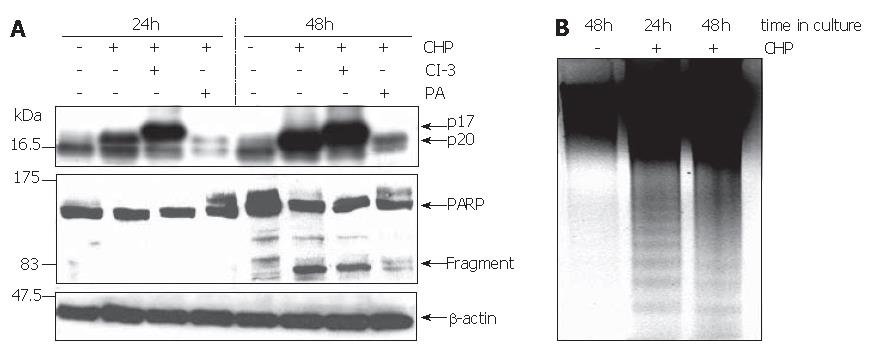
The reduction in cell viability pointed to the induction of apoptosis by CHP. Using the Western blot technique, we analyzed the processing of caspase-3 and poly-(ADP ribose) polymerase (PARP) to verify programmed cell death. The activation of apoptosis-executing caspase-3 occurs in a two-step proteolytic process. Initially, the procaspase-3 is cleaved into the subunits p12 and p20, followed by an autocatalytic fragmentation of the latter one, resulting in the fully active p17 enzyme[23]. Treatment of DSL6A cells with CHP caused a time-dependent activation of caspase-3 represented by the p17 molecule (Figure 7A). Similarly, the cleaved PARP protein of 85 ku occurred during CHP treatment (Figure 7A). Intriguingly, the CHP-induced apoptotic DSL6A cell death, determined by the activation of caspase-3 and PARP, could be prevented by pre-treatment of the cells with the broad spectrum protease inhibitor PA (Figure 7A). These results together with the prevention of CHP-induced FAK degradation (Figure 3B) as well as cyclin D1 reduction (Figure 2C) by inhibition of intracellular proteolysis indicate a superior role of proteases in the CHP-mediated effects.
The caspase-3-specific inhibitor Z-DVED-FMK (CI-3) did not prevent the initial cleavage of procaspase-3 into the p20 fragment. Therefore, corresponding with the blockade of the subsequent autocatalytic processing generating the active p17 enzyme, the level of the p20 protein increased under the treatment with Z-DVED-FMK (Figure 7A).
Another characteristic sign of apoptosis is the degradation of genomic DNA into multiple fragments referred to as DNA laddering. Electrophoretical separation of DNA isolated from DSL6A cells treated for 24 h and 48 h with CHP exhibited the typical DNA ladder, whereas DNA degradation was not detected in untreated controls (Figure 4C). Interestingly, there was no effect of CHP on the anti-apoptotic factor bcl-2 (data not shown).
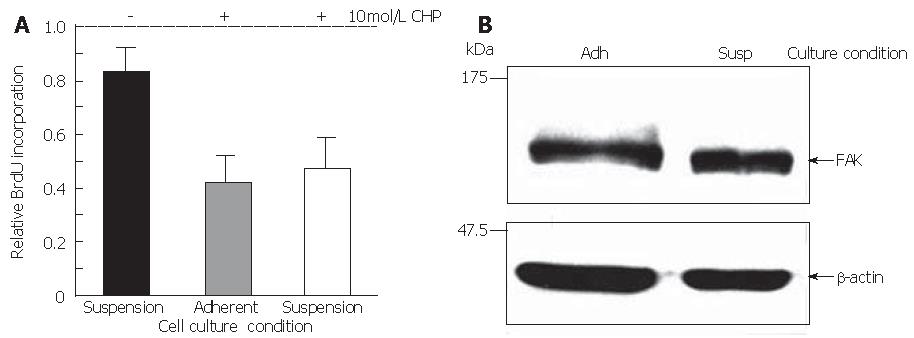
To verify the hypothesis that CHP-induced cell death was not simply the consequence of the loss of adherence, cell survival was quantified in suspension cultures compared with adherently growing DSL6A cells. Indeed, the rate of metabolically active cells (17%) in suspension was lower than that in the adherent counterparts (Figure 8A). However, the portion of dead cells in monolayer cultures incubated with CHP for 24 h increased to about 60% as compared to the untreated controls. Moreover, there were no significant differences between adherent and suspension cultures in CHP-treated cells (Figure 8A). Interestingly, the FAK molecule in DSL6A cells bared from adhering remained intact (Figure 8B). Taken together, our data suggest that the CHP-mediated damage of DSL6A cell functions, including detachment, growth inhibition and apoptosis, is a consequence of molecular mechanisms initiated by intracellular proteolytic processes.
In this study, we have shown that cis-hydroxyproline initiates a stress machinery in the rat pancreatic cancer cell line DSL6A, leading sequentially to growth inhibition, loss of adherence and apoptosis. The capacity of CHP to inhibit cell proliferation is consistent with the results for fibroblasts, mammary carcinoma and retinal epithelium cells[6-8].
The CHP-induced damage of DSL6A cells was associated with proteolytic fragmentation and inactivation of FAK. Various studies have suggested that FAK is essentially implicated in integrin-mediated transfer of extracellular stimuli from the cell surface across the plasma membrane, resulting in the activation of several intracellular signalling pathways[22-24]. FAK activation by phosphorylation has been shown in a variety of cell types to be dependent on integrins binding to their extracellular ligands[27]. In addition, there are several reports regarding the regulation of FAK activity by limited cleavage of the enzyme protein. Using human platelets, Cooray et al[28] could identify calpain as the FAK-hydrolysing protease. In a model of oncogenic cell transformation by retroviral gene transfer of v-src, Carragher et al[29] showed that cell detachment is associated with calpain-dependent FAK degradation. Furthermore, focal loss of adhesion followed by apoptosis after switching off v-src is accompanied by caspase-mediated FAK proteolysis[29]. The data and the different effects of specific protease inhibitors let the authors conclude that proteolysis of FAK might be a general phenomenon associated with disassembly of focal adhesions, but can be mediated by distinct proteases under different biological conditions[29]. We have shown that in addition to FAK cleavage, the CHP-induced cell detachment, cyclin D1 decline, and caspase-3 activation in DSL6A cells could be attenuated by proteolysis inhibition. The circumvention of the different molecular and functional effects by inhibition of intracellular proteolysis suggests that protease(s) might play a superior role in the execution of CHP-mediated cell toxicity. Furthermore, CHP induced the expression of GRP78 and GADD153, both of which have been defined to indicate the UPR, a pathway of the ER stress machinery[15].
As early as 1975, Uitto et al[22] found that non-helical CHP-containing pro-collagen is retained in the ER of chick embryo tendon cells. However, the molecular consequences of these observations could not fully be realised at this time. The CHP-initiated impairment of DSL6A cell functions might be likely attributed to more comprehensive cellular mechanisms rather than exclusively to the disturbance of collagen synthesis, especially because DSL6A cells do not express collagen type I but only traces of collagen type IV-specific transcripts (data not shown). Thus, an interference of CHP with the synthesis of proline-rich proteins involved in striking regulatory cell functions can be assumed[31].
L-proline is able to abolish CHP-induced disturbances of DSL6A cell functions. The mutual competitive replacement of the isomeric compounds proline and CHP has to be expected[5,8]. More interestingly, the addition of L-proline could arrest and even reverse the ongoing process of the CHP-induced ER stress. The restoration is accompanied by a decrease of the expression of the stress proteins, indicating the reversibility of the UPR. GRP78 transcript levels could be achieved during about 6 h of the starting status, while the normalization of the protein concentration takes more than 2 d. According to reports by Liu
et al[32], the delayed protein decline is probably attributed to its half life time of more than 36 h.
Intriguingly, the L-proline-induced reversion of the CHP-mediated impairment of cell functions is accompanied by a time-dependent reconstitution of the FAK molecule. Furthermore, the survival of DSL6A cells maintained in suspension cultures suggests that the proteolytic FAK degradation is not resulted from apoptosis, but occurs downstream or even constitutes a component of the ER stress cascade. In addition to the proteolytic fragmentation of FAK shown by several studies[30,29], there are reports describing ER stress-mediated activation of caspases, leading to apoptotic cell death[33]. In contrast to these data, we showed that CHP-caused DSL6A cell injury could not be influenced by inhibition of caspases, suggesting that other and/or additional protease(s) trigger CHP-induced proteolytic activity. In this context, reports should be noted which suggest the existence of a physiological role of caspases, apart from that of death executioners[33].
Based on our data, we hypothesize that CHP initiates a signal cascade including sequentially the induction of ER stress, activation of protease(s) that catalyse the caspase-independent FAK fragmentation, followed by cell detachment and finally leading to apoptosis. Further investigations are necessary to identify proteases involved in the CHP-mediated mechanisms.
Interestingly, CHP-caused FAK inactivation leading to detachment of DSL6A cells is not directly associated with apoptosis, in general the cellular response to the loss of anchorage referred to as anoikis[34]. This anoikis-resistance of the DSL6A cells can be attributed most likely to their malignant phenotype[35].
Taken together, we have shown, probably for the first time, an ER stress-mediated cytotoxic effect exerted by CHP in pancreatic cancer cells. Since the molecular equipment may vary between different cell populations, further studies are needed to characterize the susceptibility of other cell types to CHP. Lastly, in order to test the practicability of CHP in cancer therapy, studies on animal models are under way.
The authors thank Edith Prestin for the technical assistance. We are grateful to Peter Lorenz (Inst. of Immunology, University of Rostock) for excellent assistance with the laser scanning microscopy.
| 1. | Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 851] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 2. | Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001;98:2603-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 573] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690-7694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 216] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Rosenbloom J, Prockop DJ. Incorporation of cis-hydroxyproline into protocollagen and collagen. Collagen containing cis-hydroxyproline in place of proline and trans-hydroxyproline is not extruded at a normal rate. J Biol Chem. 1971;246:1549-1555. [PubMed] |
| 5. | Uitto J, Prockop DJ. Incorporation of proline analogs into procollagen. Assay for replacement of imino acids by cis-4-hydroxy-L-proline and cis-4-fluoro-L-proline. Arch Biochem Biophys. 1977;181:293-299. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Lewko WM, Liotta LA, Wicha MS, Vonderhaar BK, Kidwell WR. Sensitivity of N-nitrosomethylurea-induced rat mammary tumors to cis-hydroxyproline, an inhibitor of collagen production. Cancer Res. 1981;41:2855-2862. [PubMed] |
| 7. | Tan EM, Ryhänen L, Uitto J. Proline analogues inhibit human skin fibroblast growth and collagen production in culture. J Invest Dermatol. 1983;80:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Yoo JS, Sakamoto T, Spee C, Kimura H, Harris MS, Hinton DR, Kay EP, Ryan SJ. cis-Hydroxyproline inhibits proliferation, collagen synthesis, attachment, and migration of cultured bovine retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:520-528. [PubMed] |
| 9. | Aplin AE, Howe A, Alahari SK, Juliano RL. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol Rev. 1998;50:197-263. [PubMed] |
| 10. | Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 847] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | Beviglia L, Golubovskaya V, Xu L, Yang X, Craven RJ, Cance WG. Focal adhesion kinase N-terminus in breast carcinoma cells induces rounding, detachment and apoptosis. Biochem J. 2003;373:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Hungerford JE, Compton MT, Matter ML, Hoffstrom BG, Otey CA. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J Cell Biol. 1996;135:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 292] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Shen X, Zhang K, Kaufman RJ. The unfolded protein response--a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat. 2004;28:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1776] [Cited by in RCA: 1818] [Article Influence: 67.3] [Reference Citation Analysis (9)] |
| 15. | Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935-25938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 469] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389-1398. [PubMed] |
| 17. | Lehnert L, Lerch MM, Hirai Y, Kruse ML, Schmiegel W, Kalthoff H. Autocrine stimulation of human pancreatic duct-like development by soluble isoforms of epimorphin in vitro. J Cell Biol. 2001;152:911-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Sparmann G, Hohenadl C, Tornøe J, Jaster R, Fitzner B, Koczan D, Thiesen HJ, Glass A, Winder D, Liebe S. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G211-G219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Sparmann G, Behrend S, Merkord J, Kleine HD, Graser E, Ritter T, Liebe S, Emmrich J. Cytokine mRNA levels and lymphocyte infiltration in pancreatic tissue during experimental chronic pancreatitis induced by dibutyltin dichloride. Dig Dis Sci. 2001;46:1647-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Caspersen C, Pedersen PS, Treiman M. The sarco/endoplasmic reticulum calcium-ATPase 2b is an endoplasmic reticulum stress-inducible protein. J Biol Chem. 2000;275:22363-22372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Brewer JW, Hendershot LM, Sherr CJ, Diehl JA. Mammalian unfolded protein response inhibits cyclin D1 translation and cell-cycle progression. Proc Natl Acad Sci U S A. 1999;96:8505-8510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Uitto J, Hoffman H, Prockop DJ. Retention of nonhelical procollagen containing cis-hydroxyproline in rough endoplasmic reticulum. Science. 1975;190:1202-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Han Z, Hendrickson EA, Bremner TA, Wyche JH. A sequential two-step mechanism for the production of the mature p17: p12 form of caspase-3 in vitro. J Biol Chem. 1997;272:13432-13436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 197] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Abbi S, Guan JL. Focal adhesion kinase: protein interactions and cellular functions. Histol Histopathol. 2002;17:1163-1171. [PubMed] |
| 25. | Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701-706. [RCA] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 853] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 26. | Ilić D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401-407. [PubMed] |
| 27. | Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1264] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 28. | Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem J. 1996;318:41-47. [PubMed] |
| 29. | Carragher NO, Fincham VJ, Riley D, Frame MC. Cleavage of focal adhesion kinase by different proteases during SRC-regulated transformation and apoptosis. Distinct roles for calpain and caspases. J Biol Chem. 2001;276:4270-4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Buday L, Wunderlich L, Tamas P. The Nck family of adapter proteins: regulators of actin cytoskeleton. Cell Signal. 2002;14:723-731. [RCA] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Liu H, Bowes RC, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751-21759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 302] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Momoi T. Caspases involved in ER stress-mediated cell death. J Chem Neuroanat. 2004;28:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 33. | Zeuner A, Eramo A, Peschle C, De Maria R. Caspase activation without death. Cell Death Differ. 1999;6:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2335] [Cited by in RCA: 2475] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 35. | Grossmann J. Molecular mechanisms of "detachment-induced apoptosis--Anoikis". Apoptosis. 2002;7:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
S- Editor Wang J L- Editor Kumar M E- Editor Bi L