Published online Mar 7, 2005. doi: 10.3748/wjg.v11.i9.1251
Revised: July 20, 2004
Accepted: September 9, 2004
Published online: March 7, 2005
Cancer development is essentially a tissue remodeling process in which normal tissue is substituted with cancer tissue. A crucial role in this process is attributed to proteolytic degradation of the extracellular matrix (ECM). Degradation of ECM is initiated by proteases, secreted by different cell types, participating in tumor cell invasion and increased expression or activity of every known class of proteases (metallo-, serine-, aspartyl-, and cysteine) has been linked to malignancy and invasion of tumor cells. Proteolytic enzymes can act directly by degrading ECM or indirectly by activating other proteases, which then degrade the ECM. They act in a determined order, resulting from the order of their activation. When proteases exert their action on other proteases, the end result is a cascade leading to proteolysis. Presumable order of events in this complicated cascade is that aspartyl protease (cathepsin D) activates cysteine proteases (e.g., cathepsin B) that can activate pro-uPA. Then active uPA can convert plasminogen into plasmin. Cathepsin B as well as plasmin are capable of degrading several components of tumor stroma and may activate zymogens of matrix metalloproteinases, the main family of ECM degrading proteases. The activities of these proteases are regulated by a complex array of activators, inhibitors and cellular receptors. In physiological conditions the balance exists between proteases and their inhibitors. Proteolytic-antiproteolytic balance may be of major significance in the cancer development. One of the reasons for such a situation is enhanced generation of free radicals observed in many pathological states. Free radicals react with main cellular components like proteins and lipids and in this way modify proteolytic-antiproteolytic balance and enable penetration damaging cellular membrane. All these lead to enhancement of proteolysis and destruction of ECM proteins and in consequence to invasion and metastasis.
- Citation: Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol 2005; 11(9): 1251-1266
- URL: https://www.wjgnet.com/1007-9327/full/v11/i9/1251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i9.1251
The conversion of normal cells into invasive cancers with metastatic potential is a process that involves several steps. These steps are manifested in distinguishable histological and temporal stages; for instance, normal tissue, hyperplasia with a high incidence of proliferating cells, dysplasia with the induction of angiogenesis before the emergence of frank tumors with metastatic potential. Analysis of the latter stages of tumor progression has resulted in a multi-step theory of carcinogenesis on the basis of genetic changes involving activation of oncogenes, inactivation of tumor suppressor genes and altered expression of tumor-associated molecules.
Requisite for neo-plastic cell and capillary or inflammatory cell invasion during carcinogenesis processes is the remodeling events that occur within the stroma or extracellular matrix (ECM). The ECM is a complex meshwork of collagens, fibrillar glycoproteins and proteoglycans that determines tissue architecture and conditions many biological activities. The ECM is involved in both normal and pathological processes. Components of the ECM provide a large variety of specific signals that directly influence cell proliferation, migration, morphology, differentiation, and biosynthetic activities[1]. In addition, ECM plays an essential role in cell survival, since loss of adhesive contact, results in apoptosis termed anoikis[2]. In tumors, alterations of the ECM might therefore lead to abnormal host and cancer cell functions and even to cancer progression. Perturbations in the production, deposition and degradation of matrix components have been observed in mammary carcinoma[3]. Quantitative changes in matrix components may be related to an imbalance between their synthesis and degradation. Tumor cells may directly alter the adjacent matrix by changing the production of matrix proteins or proteolytic enzymes. Alternatively, the desmoplastic response may depend on specific interactions between tumor cells and host fibroblastic cells.
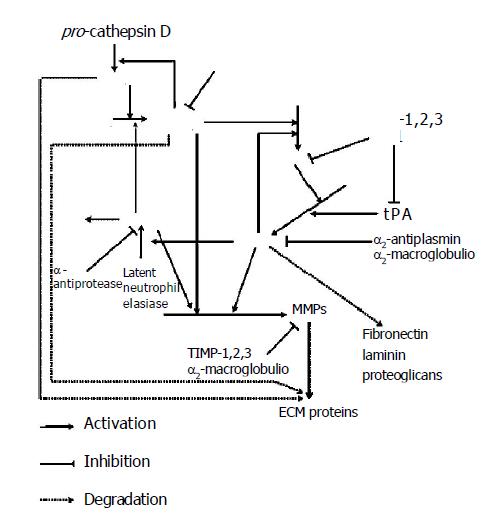
It is suggested that perturbation of the tissue microen-vironment may be sufficient to induce tumor formation. Moreover, tumor cell invasion and metastasis also require destruction of the ECM during local invasion, angiogenesis, intravasation and extravasation. These processes are mediated by multiple degradative actions of proteolytic enzymes and these complex events need cooperation of different specificity proteases. There are four major groups of endoproteases: the aspartyl and cysteine enzymes (mainly cathepsins), which function at low pH and are involved mainly in intracellular proteolysis within lysosomes and serine and metal-dependent enzymes, which are active at neutral pH and responsible for extracellular proteolysis[5]. These enzymes can act directly by degrading ECM or indirectly by activating other proteases, which then degrade the ECM. They act in a determined order, resulting from the order of their activation. When proteases exert on other proteases, the end result is a cascade leading to proteolysis. Presumable order of events in this complicated cascade is that procathepsin B can be activated by cathepsin D and other proteases[6]. Once activated, cathepsin B may play an important role as an activator of other proteases. For example, active cathepsin B can activate pro-uPA, which is secreted as an inactive proenzyme[7]. Then active uPA can convert plasminogen into plasmin. However, cathepsin B and plasmin are capable of degrading several components of tumor stroma and may activate zymogens of matrix metalloproteinases (MMPs) (Figure 1)[8].
In the result of the above reactions, the first glycoproteins are degraded by cathepsins and plasmin and afterwards collagen is degraded by metalloproteinases. This order is a result of ECM composition. Glycoproteins surround collagen structures and protect them against proteolysis. Removing glycoproteins by plasmin enables collagen degradation by collagenases. The degradation of these components destabilizes ECM structure and enables neoplastic cells to migrate. Normal and neoplastic cells adhere to adhesive proteins localized on cell membrane surface junctions. There are also cell adherence to cell and cell to ECM adherence. Detachment of neo-plastic cells from the tumor is enabled by action of proteolytic enzymes degrading these proteins[9]. Free neo-plastic cells migrate in dense mesh of ECM due to proteolytic enzymes. Neo-plastic cells that cannot change their shape easily are particularly difficult to pass through ECM. The basement membrane also stands in the way of migrating neo-plastic cells. The main component of the basement membrane is collagen type IV. Leukocyte proteases participate in the process of protease activation and facilitate neoplastic cells passing through the basement membrane[10]. The contact of leukocytes with neoplastic cells enhances the synthesis of other proteolytic enzymes. Consequently, common action of different substrate specificity and localization in protease activation processes are completed and degradation of the ECM proteins and the basement membrane contribute to cancer development.
Activation of cascade proteases participating in ECM degradation, in general, is induced by acidic proteases that are named cathepsins and collected mainly in lysosomes. In normal cells, only a small (5-10%) quantity of cathepsins occurs in cytosol. However, tumor promotion is connected with disturbances in post-translational glycolization and phosphorylation of proenzymes in Golgi apparatus that prevents their transport to lysosomes. Consequently, a great majority of these proteases occur in cytosol of neoplastic cells[11]. The biological task of cathepsins is to degrade cellular and extracellular proteins and cathepsins, with different specificity, are complementary in their action on proteins. They cause total proteolysis degrading proteins of ECM and basement membrane components, as well as, participate in limited proteolysis, e.g., activating proenzymes and prohormones[12-14]. Aspartyl protease cathepsin D and many cysteine proteases participate in tumor development.
Cancer cells are characterized by over-expression and secrete a large proportion of cathepsin D[15]. Over-expression of cathepsin D has been described both at mRNA and at protein levels[16-18]. Cathepsin D is synthesized in an inactive form as procathepsin D, which has no proteolytic activity[19]. It is auto-activated in an acidic environment (pH<5) in acidic intracellular vesicles or activated during the action of other proteases[20]. Secreted procathepsin D could also be activated extracellularly in sufficiently acidic milieu. The extracellular pH in tumors is generally more acidic than that in corresponding normal tissue[21]. Above transformations lead to the formation of an active mono-chain form and then to the formation of an active bi-chain form. No difference has been shown in the composition and amino acid sequence of cathepsin D in normal and neoplastic tissues. The observed qualitative differences concern only the enzyme structure depending on post-translation processes[22]. But there are differences in distribution of cathepsin D, i.e., it is found not only in the lysosomes of cancer cells but also in the cytosols as well[23-25]. The significant increase in cytosols and in the neo-plastic cells has been shown in cases of breast cancer[26]. Moreover, the correlation between high cathepsin D expression in cytosols and poor survival in node-negative breast cancer is observed[27,28]. Marked expression of cathepsin D takes place in carcinomas of uterus, ovary, lung, intestines and many other organs[24,26,29-31]. Related increase of cathepsin D activity and concentration is also observed in the fluids of the body cavities and in the blood serum of patients with carcinomas[32-37].
Cathepsin D as an endopeptidase degrades many intracellular and endocytosed proteins, as well as, ECM proteins and proteins of the basal epithelium. Cathepsin D participates in limited proteolysis and activates cysteine procathepsins B and L; it also degrades and makes inactive their active forms[6]. In addition, it inactivates cysteine proteases inhibitors - cystatins[38]. It has been shown that human cathepsin D stimulates tumor growth by acting directly or indirectly as a mitogenic factor on cancer cells independently of its catalytic activity[39]. Cathepsin D stimulates proliferation and tumor angiogenesis of cancer cells. It was suggested that cathepsin D stimulates angiogenesis by releasing ECM-bound bFGF or pro-cathepsin D is responsible for generation of specific inhibitor of angiogenesis angiostatin[12,40]. Now it is speculated that cathepsin D may stimulate endothelial cell growth via a paracrine loop, acting as a protein ligand, by directly or indirectly triggering a yet unidentified cell surface receptor[39,41]. Moreover, it has been documented that cathepsin D influences apoptosis and mediates apoptosis induced in cell lines by various agents, i.e., INF-γ, Fas/APO, FNF-α and oxidative stress[42,43]. However, now it is suggested that proteolytic action of cathepsin D may provide some protection against apoptosis[39]. Thanks that it participates in the process of neo-plastic growth and metastasis. Moreover, until now an endogenous cathepsin D inhibitor has not been found, the reason why it can work in each condition is still unknown.
The second step in proteolytic cascade may be linked with different lysosomal cysteine proteases, including cathepsins B, L, H, C, S, F, K, O, V, W and X[44]. The promotion role of these cathepsins is connected with their proteolytic effects on basement membrane and interstitial stroma[45]. In normal cells, these cathepsins are regulated at every level of their biosynthesis including transcription, post-transcriptional processing, translation, post-translational processing and trafficking, thus maintaining their normal function in cell metabolism. In tumor cells, misregulation of cathepsins at one or more of these levels results in increased mRNA and protein expression, increased activity and altered intracellular distribution[45]. Among the lysosomal cysteine proteases, cathepsin B has been the most extensively investigated due to its important role in cancer progression[45-48].
Increased expression together with enhanced secretion and cell surface association of cathepsin B is found in different types of tumor cells, especially in their more malignant variants[25,49,50]. Studies have revealed that enhanced production and release of this cathepsin in tumor cells lead to tumor cell growth, invasion and metastasis[51,52].
It has been found that cathepsin B mRNA, protein level and activity in tumors and cancer cell lines, especially with high metastatic potential, are increased[48]. Over-expression of cathepsin B mRNA has been reported in several human tumors including tumors of brain, colon, prostate and thyroid[52-55]. However, increased expression of cathepsin B, in pre-malignant lesions, suggests that this enzyme may play a role in the transformation of pre-malignant lesions to malignant tumors[56]. Moreover, it has been found that cathepsin B expression often increases specifically at the invasive edge of tumor cells[53], because granules containing cathepsin B, in normal tissues, are localized perinuclear while during tumor progression they move to the inner basal surface of plasma membrane[57]. The redistribution of cathepsin B to the basal membrane in cancer cells occurs coincidently with degradation of the underlying basement membrane. It is very important for degradation of the surrounding ECM in tumor progression, because tumor cell invasion involves local proteolysis.
Although, a few studies indicate a correlation between cathepsin B mRNA over-expression and tumor invasion, numerous studies have been focused on cathepsin B expression at the level of protein and activity. Cathepsin B protein and activity levels have been found to be higher in many human tumors including tumors of breast, cervix and ovary, colon, stomach, glioma, lung and thyroid[48,58,59].
Cathepsin B like other cathepsins is synthesized as a proenzyme and activated in prelysosomal acidic vesicles prior to its delivery to lysosomes. Procathepsin B can be activated by cathepsin D, elastase and cathepsins G, uPA or tPA[6]. Mature cathepsin B is localized in lysosomal and participates in intracellular proteolysis. However, in cancer cells significant increase of cathepsin B in cytosol has been observed[60,61]. Increased secretion of proenzymes, as well as, mature enzymes by human colorectal carcinoma cell lines and hepatomas and human liver, colorectal and lung cancers have been reported[62-64]. Moreover, secretion of procathepsin B can occur from cells that do not exhibit an increase in mRNA levels, indicating that this secretion is probably due to altered intracellular trafficking and distribution of this enzyme[65]. Another indication that tumor cells secrete cathepsin B is the increased serum level of cathepsin B in patients with hepatocellular carcinoma, prostate cancer and melanoma[66-68]. In addition, cathepsin B has also been found in other body fluids surrounding tumors, such as bronchoalveolar lavage fluid of lung cancer patients or cerebrospinal fluid from patients with leptomeningeal metastasis[69,70].
Cathepsin B can affect extra-cellular connective matrix directly causing its proteolytic degradation or indirectly via activation or amplification of other ECM-degrading proteases. This cathepsin may act on the contact regions of tumor cells and basement membrane or interstitial stroma. These places are often acidified by tumor cells, that are conducive to activation of secreted precursors to active forms that degrade the protein components of basement membranes and the interstitial connective matrix including laminin, fibronectin, elastin, and various types of collagen[71,72]. Digestion of fibronectin by cathepsin B results in exposure of the CS-1 sequence, which is within the alternatively spliced type III connecting segment (IIICS) of fibronectin and recognized by the integrin receptor, α4β1[73]. In this way, cathepsin B may not only be involved in extra-cellular degradation but may also be linked to cellular signal transduction events.
Cathepsin B indirectly enhances proteolysis by activating the urokinase-type pro-plasminogen which can subsequently activate the plasmin-metalloproteinases proteolytic pathway[74,75]. Moreover, cathepsin B may change the balance between metalloproteinases and their inhibitors and directly activates some of the MMPs - interstitial procollagenase (proMMPs-3) and prostromelysin-1 (proMMPs-2)[76,77], directly cleaves and inactivates some of the MMP inhibitors TIMP-1 and TIMP-2[78]. In such a way, cathepsin B assists tumor cells in their detachment from ECMs and metastasis. Moreover, during proteolytic breakdown of ECMs, some ECM-bound growth factors such as bFGF, EGF, TGF-β, IGF-I and VEGF may be liberated and become bioavailable for growth modulation of receptor-partner expressing tumor and stroma cells[79,80].
It has been proved that another cysteine protease, cathepsin L, has similar proteolytic properties and action in tumor progression to cathepsin B[81]. The highest level of cathepsin L is seen in most of the tumors and transformed cell lines possessing the highest malignancy[82]. The cathepsin L gene may be activated by a variety of growth factors and activated oncogenes[83]. It has also been shown that procathepsin L possesses proteolytic activity and can act like a growth factor or a progression factor on cell proliferation and is involved in differentiation processes[83,84]. Moreover, procathepsin L degrades both fibronectin and laminin while cathepsin L degrades types I and IV collagens, fibronectin and laminin.
Other cysteine proteases like cathepsins H and K participate in carcinogenesis. Cathepsin H is easily distinguished from other endosomal cysteine proteases by its unique aminopeptidase activity[85]. There is growing evidence that the expression of cathepsin H increases in disease states including breast carcinoma, melanoma, glioma, and lung, colorectal, prostate and other carcinomas[11,60,86-90]. In contrast to cathepsins B and L, it seems that the over-expression of cathepsin H does not strongly correlate with the malignancy of tumors or transformed cells[82]. Significant increase in serum of patients with metastatic melanoma and significant decrease in head and neck tumor tissues have been observed[91]. The role of cathepsin H in tumor progressions is not well understood. A possible function of cathepsin H in tumor progression is its ability to degrade fibrinogen and fibronectin, suggesting that along with other proteases, cathepsin H may be involved in the destruction of ECM components leading to cancer proliferation, migration and metastasis[92]. Cathepsin K has a strong collagenolytic and elastinolytic activity and may effectively degrade ECMs[93,94]. The expression of cathepsin K is enhanced at mRNA level and even at protein level in breast, lung and prostate cancers[95,96]. The activity of lysosomal cysteine proteinases is controlled by inhibitors existing in tissues, blood plasma and other body fluids, e.g., cystatins, stefins, kininogen and α2-macroglobulin[87,97,98].
Proteolytic activities of cathepsins B and L are inhibited by cystatin family including secretory cystatins C, E/M, F and stefins A and B[99-102]. The strongest inhibitory effect on cysteine proteases demonstrates that cystatin C also inhibits activity of cathepsins K and H[87,103]. Distribution of particular inhibitors in organism is different. Stefins A and B exist mainly in tissues whereas cystatin C and kininogen in blood plasma and other body fluids[99]. The activity and concentration of these inhibitors are changed in many pathological conditions including cancer[104]. A decrease of cystatin concentration results in the increase of cysteine cathepsin activity and enhancement of pathological process. It has been demonstrated that cystatins can restrain tumor cell invasion and metastasis[105]. In some regions of tumor cells, with mild acidic pH, the activity of secreted cathepsin B may also be inhibited by cysteine[106]. However, in the regions of tumor cells, where the pH is neutral or slightly alkaline, the secreted active cathepsins may undergo irreversible inactivation[107-109]. In the presence of acidic glycosaminoglycans the process of pH-induced inactivation of cathepsins can be significantly slowed down[108,110].
During the past decades, the important role of ECM, in indirect digestion, has been attributed to plasminogen activator system, which is composed of pro-activators, plasminogen, their cell surface receptors and activation inhibitors and antiplasmins. There are two types of plasminogen activators: the urokinase-type (uPA) and the tissue type (tPA). These activators are coded by two different genes[111]. However, they are quite similar in structure. Both are capable of catalyzing the conversion of inactive zymogen plasminogen to active proteinase plasmin. There is evidence that the primary role of tPA is to generate plasmin for thrombolysis, while it is the uPA that generates plasmin in events involving degradation of ECM. In consequence uPA participates in cancer invasion and metastasis[112]. uPA is released from cells as a zymogen form of pro-uPA, which is converted to active form by plasmin, present in trace quantities in ECM. Elastase and cathepsin B are also pro-urokinase activators[7]. UPAR, a surface receptor, binds to pro-uPA with high affinity[113]. Coincident binding of pro-uPA and plasminogen to uPAR and free lysine groups, on cell surfaces strongly enhance plasminogen activation[114]. This situation facilitates pro-uPA by plasmin and activation of plasminogen by urokinase. Because plasmin is produced in the second reaction, the activation behaves like a closed cycle reaction. Two inhibitors control plasminogen activation. There are two main inhibitors of plasminogen activators, PAI-I and PAI-2, while plasmin is inhibited by α2-antiplasmin[115]. These inhibitors belong to the serpin super-family. The other serpins, proteinase nexin-1 (PN-1) and protein C inhibitor can also inhibit uPA and tPA at physiologically relevant rates, though they are not specific for plasminogen activators and react more slowly with these proteases than PAI-1 and PAI-2[116,117]. Active plasmin can degrade most ECM proteins such as fibronectin, vitronectin and fibrin, a notable exception being native collagens[118]. It can also indirectly promote matrix degradation through activation of some but not all pro-metalloproteinases[77]. Plasmin probably has functions unregulated in matrix degradation, e.g., activation of proforms of cytokines and growth factors such as pro-TGF-β[119]. In addition, activation of proteolysis by plasminogen activator system has been reported in several human malignancies and is believed to contribute to tumor cell mobility and invasion[120,121].
Metalloproteinases (MMPs) are a family of secreted or transmembrane proteins that are capable of digesting ECM and basement membrane components under physiological conditions. Based on their structure and substrate specificity, they can be classified into different groups of closely- related members. Collagenases degrade fibrillar collagen; gelatinases are particularly potent in degradation of nonfibrillar and denatured collagen (gelatin), stromelysins prefer proteoglycans and glycoproteins as substrates, membrane-type MMPs (MT-MMPs) and others[122-124] (Table 1). They share a catalytic domain with the HEXGH motif responsible for ligating zinc, which is essential for catalytic function[75]. In contrast to soluble MMPs, MT-MMPs possess a transmembrane domain at their COOH terminus, resulting in cell surface localization[125]. MMPs are also characterized by a distinctive PRCGVPD sequence in the prodomain that is responsible for maintaining latency in the zymogens. MMP family members differ from each other in structure by the presence or absence of additional domains that contribute to activities, such as substrate specificity, inhibitor binding, matrix binding and cell-surface localization[126].
| Protease family | Protease | Protease function | Protease inhibitors |
| Aspartyl protease | Cathepsin D | Degradation of ECM components | |
| Conversion of cysteine procathepsins into cathepsins | |||
| Cysteine proteases | Cathepsins B, L, H, K | Degradation of ECM components | Cystatins, stefins, kininogen |
| Conversion of pro-MMPs into MMPs | |||
| Serine proteases | Plasmin | Degradation of ECM components | α2-antiplasmin, |
| Activation of uPA | α2-macroglobulin | ||
| Conversion of inactive elastase into elastase | |||
| Urokinase-type plasminogen activator (uPA) | Conversion of plasminogen into plasmin | PAI-1, 2, 3 | |
| Tissue-type plasminogen activator (tPA) | Conversion of plasminogen into plasmin | ||
| Neutrophil serine proteases | Elastase | Degradation of ECM components | α2-antiplasmin |
| Cathepsin G | α2-macroglobulin | ||
| secretory leukoprotease inhibitor | |||
| Matrix metalloproteinases | Degradation of collagens and other ECM proteins | TIMP-1, 2, 3, 4 | |
| Activation another pro-MMPs into MMPs | α2-macroglobulin | ||
| Degradation of collagens: I, II, III, VII, X and | |||
| Collagenases [MMP-1, 8, 13] | gelatins | ||
| Degradation of proteoglycans, laminin, gelatins, | |||
| Stromelysins [MMP-3, 10] | collagens III, IV, V, IX, fibronectin, entactin, SPARC, | ||
| collagenases-1 | |||
| Degradation of gelatins, collagens: I, IV, V, VII, X, | |||
| Gelatinases [MMP-2, 9] | fibronectin, elastin, procollagenase-3 | ||
| Degradation of collagen I, II, III, gelatins, aggrecan, | |||
| fibronectin, laminin, vitronectin, MMP-2,13, tenascin, | |||
| Membrane-type [MMP-14, 15, 16, 17, 24, 25] | nidogen | ||
| Degradation of proteoglycans, laminin, fibronectin, | |||
| gelatins, collagens IV, elastin, entactin, tenascin, a1 | |||
| Others [MMP-7, 11, 12, 19, 20, 23] | -antiproteinase, amelogenin | ||
MMPs are generally produced by cells at very low levels. However, when either physiological or pathological circumstances require remodeling of ECM, increased expression levels of the enzymes can be induced[127]. The activity of MMPs is tightly regulated by the action of activators or proenzymes and inhibitors. Transcriptional regulation of MMP genes is mediated by an AP-1 regulatory element in their proximal promote region[128]. MMP gene transcription is induced by a variety of extracellular stimuli, such as cytokines (IL-4 and IL-10), growth factors such as TGF-α, bFGF, and TGF-β and cell-cell or cell-matrix interactions[129-135]. Binding of these stimulatory ligands to their receptors triggers a cascade of intracellular reactions that are mediated through at least three different classes of mitogen-activated protein kinases: extra-cellular signal-regulated kinase (ERK), stress-activated protein kinase/Jun N-terminal kinases (SAPK/JNKs) and p38[136,137]. Activation of these kinases culminates in the activation of a nuclear AP-1 transcription factor, which binds to the AP-1 cis element and activates the transcription of the corresponding MMP gene[138]. Next to the AP-1 element, other transcription factors such as ETS are involved in the regulation of MMP gene expression during tumor cell invasion[137]. Expression of ETS-1 has been demonstrated in stromal fibroblasts adjacent to invading tumor cells and in endothelial cells during tumor vascularization[139]. In general, the ERK1/2 cascade is activated by mitogenic signals, resulting in phosphorylation of various substrates including Elk-1 and subsequent activation of c-fos transcription. SAPK/JNKs and p38 are activated by cytokines (THF, IL-1) and cellular oxidative stress resulting in phosphorylation of c-Jun and ATF-2, which then induce c-jun transcription[140,141]. Constitutively active mutants of Raf-1 and MEK1 transform fibroblasts in vitro and in vivo, activation of the ERK1/2 pathway has been observed in renal and breast carcinomas[142,143]. However, blocking the pathway ERK and ERK 2, by a specific chemical inhibitor PD98059, inhibits the expression of MMP-1 and MMP-2 by SCC cells, and their invasion in vitro[122]. MMP expression has been shown in a variety of tumor types including carcinomas of lung, colon, breast and pancreas[3,144-148,149]. In addition, the plasma and urine levels of MMPs are elevated in patients with cancer compared with healthy subjects[150]. MMPs in tumor tissues are produced not only by malignant tumors but also by stromal fibroblasts and inflammatory cells[112]. These cells may produce cytokines and proteins that induce the production of MMPs by surrounding cells, creating extracellular networks of MMP secretion and activation[151-153]. Furthermore, analyses of cellular components derived from primary tumor tissues or their corresponding lymph node metastases demonstrated that expression of MMPs is increased in the metastatic tissue, indicating that MMP expression is a component of the metastatic process[148]. In addition, there is clinical evidence that over-production of these molecules confers a poor prognosis in patients with a variety of malignancies[154,155].
At the protein level, biological activity of MMPs is determined by the action of activators of proenzymes and inhibitors. In general, MMPs are secreted in latent forms (as proenzymes) and require specific proteolytic activation[75]. Most MMPs are activated in the extra-cellular space, with the exception of MMP-11 and MT1-MMP, which are activated prior to secretion of intra-cellularly by furin-like proteases[156]. This activation step serves as a regulatory element. MMP activity is further regulated by MMP inhibitors (TIMPs) and expression of molecules, which present the active enzyme on the cell surface[157,158]. However, most MMPs are converted into active forms in a specific multistep activation process that is known as the “cysteine switch”. A conserved unpaired cysteine residue in the prodomain forms a coordinate bond with zinc ion at the active site. Cleavage of the prodomain results in opening of the active site by disruption of the zinc–cysteine bond and is followed by loss of the amino-terminal prodomain[104]. For most MMPs, proteolytic activation is initiated in the extracellular space by serine proteases such as plasmin and urokinase plasminogen activator, neutrophil elastase or by other members of the MMP family members, especially MMP-2 and MMP-13 by MT1-MMP cleavage of the proenzymes[104,127,156]. Activation of MMPs can also be induced by non-proteolytic compounds such as thiol compounds including oxidized glutathione and hypochlorous acid[75,162]. Therefore, MT-MMP expression is another regulating factor for MMP activation[124,138]. However, little is known about the activation induced by other factors including other MMPs, cytokines, trypsin, serine proteases, reactive oxygen species, hypoxia, c-erbB ligands, and leukocyte elastase[159-164]. Some activated MMPs can further activate other proMMPs. MMPs containing a furin-like recognition domain (RXKR) in their propeptides (MMP-11, MMP23 and MT-MMPs) are activated intracellularly in the trans-Golgi network by a group of calcium-dependent transmembrane serine proteases (furin/PACE/kex-2)[124,165,166]. Proteolytically active MMPs may be localized on the cell surface by binding to membrane molecules, which leads to a more direct ECM degradation. Furthermore, functionally active MMP-2 can also bind to integrin αvβ3 while proteolytically active MMP-9 can associate with CD44[167-169].
Active MMPs are inhibited by various protease inhibitors such as non-specific protease inhibitors (α2-macroglobulin and α1-antiprotease) and a family of specific tissue inhibitors, (TIMPs)[127]. There are currently four members of the TIMP family (TIMP-1, -2, -3, -4) that have common inhibitory effect on MMPs. TIMPs share several structural similarities between each other such as the N-terminal MMP binding site, 12 cysteine residues within the conserved domain which form disulfide bonds[170,171]. TIMPs are found to be involved in connective tissue turnover and remodeling. They bind to MMPs by forming non-covalent complexes, resulting in MMP inhibition[160]. TIMPs bind to either proMMPs or active MMPs, thereby inhibiting the autocatalytic capacity of active proteases. TIMP-1 and TIMP-2 regulate activation of proMMP-9 and proMMP-2, respectively[160]. TIMP-1 inhibits the activity of most MMPs, with the exception of MT1-MMP and MMP-2. TIMP-2 also inhibits the activity of most MMPs, except for MMp-9. TIMP-3 inhibits the activity of MMP-1, -2, -3, -9 and -13 and human TIMP-4 inhibit the activity of MMP-2, -9, -7[172-175]. It is clear that balance between active MMPs and TIMPs appears to be critical for MMP activity and for ECM degradation.
The role of the MMP family in tumor development is further complicated by the balance of these proteins in relation to TIMPs. TIMPs have various antioncogenic functions and the expression of TIMPs has been associated with less aggressive tumor behavior and favorable prognosis in patients with cancer[176]. For example, the exposure of mouse fibroblasts to TIMP-1 and -2 in vitro could inhibit oncogenic transformation by oncogenic viruses, whereas administration of recombinant TIMP-1 to mice injected with B16F10 melanoma cells could reduce the number of pulmonary metastases[177]. Furthermore, transgenic mice overexpressing TIMPs display resistance to intravenously injected malignant cells. Conversely, exposure of mouse fibroblasts to TIMP-1 antisense oligonucleotides could result in the formation of metastatic tumors in nude mice given injection of malignant cells[178]. Of interest, over-expression of TIMP-3 induces apoptosis in various types of malignant cells, suggesting that TIMPs may play a role in tumor cell death[176]. Thus, the role of TIMPs in vivo is complex, and the expectation that malignant tumors increase MMP expression accompanied with decreased TIMP expression is probably too simplistic[179-182].
Apart from the above proteolytic systems participating in enhanced degradation of interstitial matrix observed during cancer development, neutrophil proteases may also take part in it[183,184]. It has been shown that in particular chronic inflammation neutrophils may create an environment that supports tumor promotion[185,186]. In such a situation proteases of accumulated neutrophils may be significant factors for ECM degradation. Neutrophil granules contain a large family of over 20 enzymes such as metalloproteinases, collagenase, gelatinase and elastase and cathepsin G, but has the greatest potential to act as a mediator of tissue destruction[187,188]. However, the ECM is protected from degradation by the major plasma protease inhibitors and there is a critical balance between the enzymes and their inhibitors[189]. α1-antiprotease, α2-macroglobulin and secretory leukoprotease inhibitors are the main plasma protease inhibitors that effectively regulate extracellular neutrophil elastase and cathepsin G[189,190]. However, proteolytic-antiproteolytic balance directly depends on reactive oxygen species (ROS) generated by activated neutrophils[191]. It is known that inflammatory process and different carcinogens stimulate phagocytic cells including neutrophils, monocytes and macrophages, which undergo a respiratory burst to generate both superoxide anion and hydrogen peroxide[192]. The source of superoxide generation in these cells is a membrane-bound NADPH oxidase that remains dormant until activated by a complex cascade of signal transduction[193,194]. The combined activities of NADPH oxidase and myeloperoxidase in phagocytes leads to the production of hypochlorous acid, one of the strongest physiological oxidants[195]. In addition, it has been documented that the generation of superoxide anion, hydroxyl radical, hydrogen peroxide, singlet oxygen and hypochlorous acid is enhanced in cancer cells[196-198]. Moreover, many exogenous chemicals can be activated to radical intermediates, which can serve as electrophiles or participate in redox cycling processes of reactive oxygen generation. In addition, both exogenous and endogenous agents can interact with various cellular receptors, such as the Ah receptor and protein kinase C leading to increased oxidation in cells[199]. In such a case ROS may disturb cellular metabolism and directly or indirectly influence proteolytic-antiproteolytic balance of cancer tissue.
It is known that ROS may indirectly act as a second messenger. Some cytokines (TNF, IL-1) and growth factors (bFGF, VEGF) are capable of producing ROS in target cells and the produced ROS further participate in mediating the effects of cytokines and growth factors[185,196,200,201]. ROS may also serve as common signaling molecules regulating the activity of transcription factors, NF-κB and AP-1, in response to cytokines and other stimuli[202-203]. Oxidants can also stimulate transcription of other transcription factor genes such as c-jun, c-fos and c-myc in various cell types[204-206]. The regulation of gene expression by these factors will ultimately lead to a series of cellular changes such as proliferation, growth suppression, differentiation, senescence and apoptosis. These transcription factors are involved either directly in the induction of the expression of MMP genes (by binding to enhancer regions in these genes) or indirectly in modulating the expression of proteins which ultimately affects proteinase release[126]. ROS may also regulate cellular components other than transcription factors.
The most important factor for proteolytic-antiproteolytic balance is that ROS influences protease activation and activity and distribution of enzymes and their inhibitors. It has been shown that ROS can activate procollagenase[207,208]. However, changes in activity and distribution of enzymes and their inhibitors are connected with direct oxidative modification of cell components.
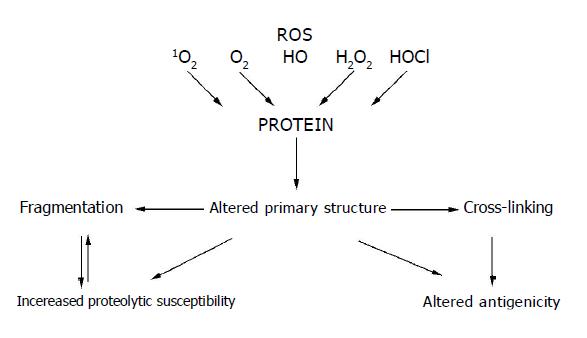
Reactive oxygen species as chemically reactive molecules can modify most cell components such as lipids, nucleic acids, carbohydrates and proteins[209]. The residues of amino acids occurring in proteins are less vulnerable to action of ROS than that of free amino acids. Most amino acids present in proteins undergo oxidative transformation to radicals, but aromatic and sulfhydryl groups containing amino acids are the most susceptible[210,211]. Susceptibility of amino acid residues to ROS also depends on their position in the polypeptide chain. Unstable amino acid radicals are formed at the first stage of ROS action and then undergo transformation yielding stable products. Other amino acid residues of proteins can also undergo oxidative transformation under formation of peroxides that are mainly generated by the aliphatic amino acid radicals[212,213]. Alteration in the primary structure of proteins brings about changes in the secondary and tertiary structures and results in denaturation, aggregation or fragmentation of protein molecules[214] (Figure 2). The protein structure modification mainly depends on the kind and concentration of acting ROS[215].
In addition, ROS can react with membrane phospholipids forming hydroperoxides and reactive small-molecule aldehydes[216] with a longer lifetime. Thus, these aldehydes can be considered as the secondary lipid peroxidation transmitters[217].
A marked increase of malondialdehyde and 4-hydroxynonenal concentrations[218,219] has been found in cancer cells. It is proved that 4-hydroxynonenal can react with sulfhydryl groups of cysteine present in polypeptide chains and with lysine, histidine, as well as other amino acid residues of proteins[217,220]. In this way, covalent lipid-membrane protein bonds are formed[221]. Lipid radicals formed by peroxidation can also react with each other forming lipid-lipid covalent bonds[221]. These reactions provoke changes in membrane structure, uncovering phosphatidylserine on its surface and modify membrane permeability[222].
An important factor preventing lipids from peroxidation is α-tocopherol[223], which reduces polyunsaturated fatty acid radicals and acts as a chain reaction terminator[224]. In addition, physicochemical action of α-tocopherol on biological membranes has a stabilizing effect and protects them from liberation and activation of endogenic phospholipases, which accelerate lipid peroxidation by hydrolyzing the lipid membrane PUFA[225]. Free fatty acids destabilize the membranes and undergo oxidation more readily than their esterified forms present in the membrane. α-Tocopherol can form more stable protective complexes with free PUFA. Stability of such complexes increases with the number of unsaturated bonds of PUFA[226]. α-Tocopherol stabilizes PUFA-rich membranes by decreasing their permeability to small ions and small molecules[225]. However, decreased α-tocopherol content in cancer tissues makes the biological membrane protection against destructive ROS action impossible[227].
The non-enzymatic mechanisms protecting cells against lipid peroxidation are completed by two GSH-dependent enzymatic systems[228]. α-Tocopherol is involved in the first system, in which a GSH-dependent α-tocopherol radical reduction to α-tocopherol takes place[229]. The second system probably consists of glutathione transferase coupled with glutathione peroxidase and reduces toxic lipid hydroperoxides to less reactive alcohols[230]. Antioxidant action of these mechanisms during cancer development may be questionable because of different data about GSH content in cancer cells[184].
A consequence of the above-discussed changes is increased fluidity of cell and lysosomal membranes. This observation is supported by transfer of lysosome enzymes to cytosol[11]. Action of ROS on cell organelle membrane components can provoke changes in function of Na+/H+ exchangers[231] possibly resulting in acidosis of the cell content, which favors the action of lysosomal proteases. These processes can bring about activation of proteolytic processes in cytosol.
The literature data concerning other experimental models confirm that ROS reactions with proteins and lipids of lysosome membranes are possible[211] and indicate that oxidation reactions catalyzed by iron ion are probable in lysosomes[232]. Lysosomes are particularly vulnerable to oxidation stress because they contain an active oxidation mediator, i.e., the iron ion. The iron ion is present in lysosomes due to endocytosis of various metalloproteins like ferritin and cytochrome and their degradation in organelles[233]. Hydrogen peroxide is formed as a result of oxidation and activation of inflammatory cells[234]. If not decomposed by catalase or peroxidase, it diffuses through the membranes to the lysosomes and takes part in intralysosome reactions catalyzed by iron ion. Hydroxyl radical is formed in such reactions and chain oxidation processes are initiated, resulting in increased permeability of the lysosome membrane or even fragmentation and liberation of the lysosome content to cytosol including proteolytic enzymes[235,236].
Oxidative modification of biologically active proteins results in changes in their activity[237]. In most cases, it results in their inactivation, particularly if the oxidative modification occurs with amino acid residues, which are essential for a specific biological activity. It was documented that cysteine proteases, in particular, are susceptible to oxidative modification[238]. On the other hand, participation of ROS is needed for some proteins to fulfill their biological function. It particularly applies to metalloproteinases, which are also activated by ROS[239].
Physicochemical and biological properties of proteins may be changed during cancer development. ROS generated, during this process, can directly react with proteins including enzymatic proteins and provoke their modification[240], which can result in activity changes of enzymatic proteins including proteases and their inhibitors.
In physiological conditions, equilibrium between proteases and their inhibitors exists in the organism. Cancer development is followed by a temporary decrease in activity of proteolytic enzymes in cancer cells with simultaneous increase in activity of proteases and decrease in activity of inhibitors in blood serum[219,241-244]. The changes mainly occur both in the enzymes containing seryl groups in their active center (cathepsin G and elastase) and in the serine protease inhibitors like α2-macroglobuline and α1-antiprotease. As the changes are temporary, it can be supposed that post-translational protein modification only occurs and its biosynthesis remains unchanged while the activity of de novo synthesized enzymes is unmodified. The modifications induced by ROS are involved in direct chemical reactions with proteases and their inhibitors. Inactivation of biologically active proteins can also be caused by their reaction with lipid peroxidation products. In the case of cathepsin B, it is the effects of thiol-ether type adduct formation by sulfhydryl groups of the active center of the enzyme and 4-hydroxynonenal[238].
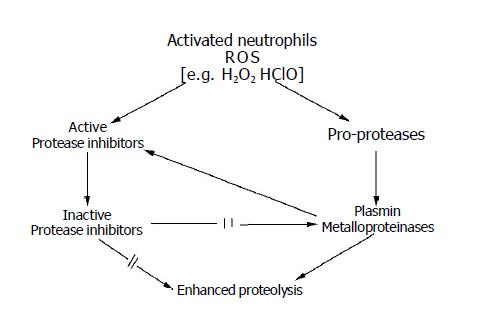
Modifications of the proteolytic enzyme structure and their inhibitors, caused by ROS, result in a shift of proteolytic-antiproteolytic equilibrium (Figure 3). However, the equilibrium perturbation enhances formation of ROS. It is considered that activation of ROS-producing granulocytes and macrophages is controlled by serine protease inhibitors[245]. decrease activity of which is observed in cancer cases[184,241-243]. Active NAD(P)H oxidase synthesized as proenzyme is necessary for ROS formation in granulocytes[191]. The activation is induced by serine protease. However, activity of this protease group is changed in the case of cancer[184,244]. In such a case, production of inhibitors of active NAD(P)H oxidase is inhibited by these enzymes and ROS production is indirectly controlled by them. Formation of superoxide anions by granulocytes is inhibited by α1-proteinase inhibitor and α1-antichymotrypsin[191,245,246]. Activity of granulocyte elastase is inhibited by hydrogen peroxide. It is suggested that another mechanism may underline the control of oxidative activation of granulocytes by inhibitors[247].
It was documented that the activity of main plasma protease inhibitors such as α1-antiproteinase and α2-macroglobulin are decreased in carcinogenesis[219,241-243]. These inhibitors as well as plasminogen activation inhibitor (PAI-1) and leukocyte protease inhibitor (SLPI) contain methionine in their inhibition center and are most susceptible to inactivation by oxidants[248,249]. Unlike them, the inhibitors containing residues of other amino acids in their inhibition center (antithrombin III, PAI-2, PAI-3) are relatively unsusceptible to oxidative inactivation[250,251]. α1-antiproteinase and PAI-1 are particularly susceptible to the action of oxidants. The α1-antiproteinase molecule contains eight methionyl residues[252]. The methionine molecule occupying the position 358 is situated in the inhibition center[253]. The action of oxidants causes oxidative modification of two methionyl residues out of eight including residues of the inhibition center[250,253]. Oxidants acting on the α1-antiproteinase molecule neither modify the radicals of other amino acids nor provoke molecule fragmentation[254]. The oxidant that probably inactivates α1-antiproteinase in in vivo conditions is chlorate (I) (ClO-) formed in myeloperoxidase-H2O2-Cl-[255]. Hydrogen peroxide generated in cancer cells can also oxidize methionine of the α1-antiproteinase molecule.
α1-antiproteinase being a polyvalent inhibitor also inhibits the activity of leukocyte cathepsin G and elastase forming inactive and stable complexes of 1:1 stechiometry[253,256]. In this way, it prevents possible damage to tissues caused by those proteases[191]. Activity of these enzymes is manifested if the mentioned inhibitor is inactivated[257]. Cathepsin G and elastase exhibit substrate specificity and can degrade elastin, collagen, proteoglycans, as well as, the complement immunoglobulin, fibrinogen, basic proteins, and other proteins. Cathepsin G is particularly aggressive in its action on proteins as it degrades them not only directly but also by activating procollagenases[258].
It has been suggested that increased activity of proteases in blood serum in cases of cancer, particularly the activity of cathepsin G and elastase, is due to increased transport from cells and reduced inactivation of inhibitors whose activity is decreased[184]. Existing protease-inhibitor inter-dependence can also suggest inactivation of inhibitors by proteases. α1-antiproteinase is known to be inactivated by sulfhydryl proteases and metalloproteinases that are also activated by ROS[259,260]. Such an action is evidenced for granulocyte collagenase[259,260], endothelium vessel stromelysine and collagenase[259,261]. These proteases split the peptide bond of Phe352 with Leu353, which are present in the α1-antiproteinase loop exposed to the exterior molecule in which the inhibition center is present. Split-out α1-antiproteinase peptide fragments are found in synovial fluid of patients with rheumatic arthritis[262], indicating that α1-antiproteinase can be inactivated both by oxidative modification and by proteolytic degradation[262,263]. Such a situation can occur particularly in the case of increased activity of proteases and has been observed in cancer development.
Changes in the proteolytic-antiproteolytic system lead to the shift of equilibrium in favor of proteases. Such a situation can lead to destruction processes because components of proteolytic-antiproteolytic system penetrate from blood to extracellular space[264] and an uncontrolled proteolysis can occur if activity of protease inhibitors is lower and activity of proteases is higher as it occurs in cancer development. It should be noted that proteolysis of ECM proteins may also be enhanced by oxidative modification of protein substrates and their increased susceptibility to protease action.
In conclusion, oxidative stress observed in tumor development may cause imbalance of proteolytic-antiproteolytic system leading to enhanced proteolysis and destruction of ECM proteins and metastasis.
The general principles of the mechanisms underlying proteolytic degradation of the ECM in cancer are becoming clear and the complexity of this process is revealed. Matrix degradation appears to be important for both cancer invasion and metastasis. It is accomplished by a cooperative interaction between several proteases. Effective protease action depends on many agents including inhibitors. The ability of TIMPs to inhibit tumor growth suggests that MMPs are more important for cancer development than normal physiological activities in adult host[127,265]. Thus, development of more specific protease inhibitors may represent tractable chemotherapeutics for human cancer. As a result, many pharmaceutical companies have designed novel drugs that variably block MMP activity. Gene delivery of TIMPs into malignant cells may also be a potent way of inhibiting the tumor invasion and in consequence prolonging survival[266]. Furthermore, an effective way of inhibiting the expression of MMPS may be blocking of signaling pathways mediating activation of MMP transcription.
Proteolytic-antiproteolytic balance also depends on the influence of other endogenous and exogenous compounds e.g., ROS, which is why the view on anti-cancer therapy pays attention to the possibilities connected with this fact. It has been mentioned above that cancer development accompanies enhanced generation of ROS, which can react with cell components and additionally influence proteolytic-antiproteolytic balance in neoplastic and surrounding cells. In this connection, approaches toward therapeutic intervention against ROS damage include administration of radical scavenger compounds, use of novel drugs that increase cellular production of constitutive antioxidants or pharmacological agents that modify the intracellular transport of antioxidants. Strategies to modify the cellular effects of ROS on cancer lead to novel approaches of gene therapy in which the antioxidant proteins can be expressed in specific tissues. Reducing tissue-damaging effects of ROS may have a relevance to cancer patients by ameliorating normal tissue damage[267].
The opposite point of view will be, prolonged accumulation of ROS is beneficial to overcoming neoplastic changes because they can kill cancer cells. Thus, inhibition of superoxide dismutase responsible for ROS elimination may provide a novel way in cancer therapy. Cancer cells are more dependent on superoxide dismutase to eliminate the toxic superoxide radicals and become more sensitive to superoxide dismutase inhibitors. In such case it is possible that inhibition of superoxide dismutase may preferentially kill malignant cells through a ROS-mediated mechanism.
| 1. | LaFlamme SE, Auer KL. Integrin signaling. Semin Cancer Biol. 1996;7:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 853] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Lochter A, Bissell MJ. An odyssey from breast to bone: multi-step control of mammary metastases and osteolysis by matrix metalloproteinases. APMIS. 1999;107:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983;49:636-649. [PubMed] |
| 5. | Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161-195. [PubMed] |
| 6. | van der Stappen JW, Williams AC, Maciewicz RA, Paraskeva C. Activation of cathepsin B, secreted by a colorectal cancer cell line requires low pH and is mediated by cathepsin D. Int J Cancer. 1996;67:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Schmitt M, Jänicke F, Moniwa N, Chucholowski N, Pache L, Graeff H. Tumor-associated urokinase-type plasminogen activator: biological and clinical significance. Biol Chem Hoppe Seyler. 1992;373:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Curran S, Murray GI. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Opdenakker G, Van Damme J. Cytokines and proteases in invasive processes: molecular similarities between inflammation and cancer. Cytokine. 1992;4:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Gabrijelcic D, Svetic B, Spaić D, Skrk J, Budihna M, Dolenc I, Popovic T, Cotic V, Turk V. Cathepsins B, H and L in human breast carcinoma. Eur J Clin Chem Clin Biochem. 1992;30:69-74. [PubMed] |
| 12. | Briozzo P, Badet J, Capony F, Pieri I, Montcourrier P, Barritault D, Rochefort H. MCF7 mammary cancer cells respond to bFGF and internalize it following its release from extracellular matrix: a permissive role of cathepsin D. Exp Cell Res. 1991;194:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Conover CA, Perry JE, Tindall DJ. Endogenous cathepsin D-mediated hydrolysis of insulin-like growth factor-binding proteins in cultured human prostatic carcinoma cells. J Clin Endocrinol Metab. 1995;80:987-993. [PubMed] |
| 14. | Siewinski M, Gutowicz J, Zarzycki A, Mikulewicz W. Role of cysteine endopeptidases in cancerogenesis. Cancer Biother Radiopharm. 1996;11:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Rochefort H. Cathepsin D in breast cancer: a tissue marker associated with metastasis. Eur J Cancer. 1992;28A:1780-1783. [PubMed] |
| 16. | Capony F, Rougeot C, Montcourrier P, Cavailles V, Salazar G, Rochefort H. Increased secretion, altered processing, and glycosylation of pro-cathepsin D in human mammary cancer cells. Cancer Res. 1989;49:3904-3909. [PubMed] |
| 17. | Cavailles V, Garcia M, Rochefort H. Regulation of cathepsin-D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol Endocrinol. 1989;3:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Rochefort H, Cavailles V, Augereau P, Capony F, Maudelonde T, Touitou I, Garcia M. Overexpression and hormonal regulation of pro-cathepsin D in mammary and endometrial cancer. J Steroid Biochem. 1989;34:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Paris A, Strukelj B, Pungercar J, Renko M, Dolenc I, Turk V. Molecular cloning and sequence analysis of human preprocathepsin C. FEBS Lett. 1995;369:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Larsen LB, Boisen A, Petersen TE. Procathepsin D cannot autoactivate to cathepsin D at acid pH. FEBS Lett. 1993;319:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64:425-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 454] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Garcia M, Platet N, Liaudet E, Laurent V, Derocq D, Brouillet JP, Rochefort H. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells. 1996;14:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Chabowski A, Skrzydlewska E, Sulkowska M, Famulski W, Zalewski B, Gnojnicki I, Kisielewski W. Cathepsin D activity in colorectal cancer. Rocz Akad Med Bialymst. 2001;46:38-46. [PubMed] |
| 24. | Chabowski A, Sulkowska M, Sulkowski M, Famulski W, Skrzydlewska E, Kisielewski W. Immunohistochemical evaluation of cathepsin D expression in colorectal cancer. Folia Histochem Cytobiol. 2001;39:153-154. [PubMed] |
| 25. | Lah TT, Kalman E, Najjar D, Gorodetsky E, Brennan P, Somers R, Daskal I. Cells producing cathepsins D, B, and L in human breast carcinoma and their association with prognosis. Hum Pathol. 2000;31:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Rochefort H, Garcia M, Glondu M, Laurent V, Liaudet E, Rey JM, Roger P. Cathepsin D in breast cancer: mechanisms and clinical applications, a 1999 overview. Clin Chim Acta. 2000;291:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Ferrandina G, Scambia G, Bardelli F, Benedetti Panici P, Mancuso S, Messori A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: a meta-analysis. Br J Cancer. 1997;76:661-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Riley LB, Lange MK, Browne RJ, Cochrane PJ, Choi IJ, Davis B, Arcona S, Alhadeff JA. Analysis of cathepsin D in human breast cancer: usefulness of the processed 31 kDa active form of the enzyme as a prognostic indicator in node-negative and node-positive patients. Breast Cancer Res Treat. 2000;60:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Oh-e H, Tanaka S, Kitadai Y, Shimamoto F, Yoshihara M, Haruma K. Cathepsin D expression as a possible predictor of lymph node metastasis in submucosal colorectal cancer. Eur J Cancer. 2001;37:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Ikeguchi M, Fukuda K, Oka S, Yamaguchi K, Hisamitsu K, Tsujitani S, Sakatani T, Ueda T, Kaibara N. Clinicopathological significance of cathepsin D expression in gastric adenocarcinoma. Oncology. 2001;61:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ioachim E, Charchanti A, Stavropoulos N, Athanassiou E, Bafa M, Agnantis NJ. Expression of cathepsin D in urothelial carcinoma of the urinary bladder: an immunohistochemical study including correlations with extracellular matrix components, CD44, p53, Rb, c-erbB-2 and the proliferation indices. Anticancer Res. 2002;22:3383-3388. [PubMed] |
| 32. | Villar C, Rodríguez JC, Raigoso PF, García-Muñiz JL, Martínez E, Vizoso F. Cathepsin D cytosol content in colorectal cancer. Int J Biol Markers. 1999;14:192-194. [PubMed] |
| 33. | Arao J, Fukui H, Ono Y, Ueda Y, Chiba T, Fujimori T. Immunohistochemical localization of cathepsin D in colorectal tumors. Dis Colon Rectum. 2000;43:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Bartenjev I, Rudolf Z, Stabuc B, Vrhovec I, Perkovic T, Kansky A. Cathepsin D expression in early cutaneous malignant melanoma. Int J Dermatol. 2000;39:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Ferrandina G, Scambia G, Bardelli F, Benedetti Panici P, Mancuso S, Messori A. Relationship between cathepsin-D content and disease-free survival in node-negative breast cancer patients: a meta-analysis. Br J Cancer. 1997;76:661-666. |
| 36. | Miyake H, Hara I, Eto H. Prediction of the extent of prostate cancer by the combined use of systematic biopsy and serum level of cathepsin D. Int J Urol. 2003;10:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Russo A, Bazan V, Migliavacca M, Zanna I, Tubiolo C, Tumminello FM, Dardanoni G, Cajozzo M, Bazan P, Modica G. Prognostic significance of DNA ploidy, S-phase fraction, and tissue levels of aspartic, cysteine, and serine proteases in operable gastric carcinoma. Clin Cancer Res. 2000;6:178-184. [PubMed] |
| 38. | Lenarcic B, Kos J, Dolenc I, Lucovnik P, Krizaj I, Turk V. Cathepsin D inactivates cysteine proteinase inhibitors, cystatins. Biochem Biophys Res Commun. 1988;154:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, Liaudet-Coopman E. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951-5955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Morikawa W, Yamamoto K, Ishikawa S, Takemoto S, Ono M, Fukushi Ji, Naito S, Nozaki C, Iwanaga S, Kuwano M. Angiostatin generation by cathepsin D secreted by human prostate carcinoma cells. J Biol Chem. 2000;275:38912-38920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H, Liaudet-Coopman E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920-6929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 1996;15:3861-3870. [PubMed] |
| 43. | Kågedal K, Johansson U, Ollinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15:1592-1594. [PubMed] |
| 44. | Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000;1477:98-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 596] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 45. | Krepela E. Cysteine proteinases in tumor cell growth and apoptosis. Neoplasma. 2001;48:332-349. [PubMed] |
| 46. | Koblinski JE, Ahram M, Sloane BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 414] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 47. | Mai J, Finley RL, Waisman DM, Sloane BF. Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells. J Biol Chem. 2000;275:12806-12812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Yan S, Sameni M, Sloane BF. Cathepsin B and human tumor progression. Biol Chem. 1998;379:113-123. [PubMed] |
| 49. | Frosch BA, Berquin I, Emmert-Buck MR, Moin K, Sloane BF. Molecular regulation, membrane association and secretion of tumor cathepsin B. APMIS. 1999;107:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Vigneswaran N, Zhao W, Dassanayake A, Muller S, Miller DM, Zacharias W. Variable expression of cathepsin B and D correlates with highly invasive and metastatic phenotype of oral cancer. Hum Pathol. 2000;31:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Dohchin A, Suzuki JI, Seki H, Masutani M, Shiroto H, Kawakami Y. Immunostained cathepsins B and L correlate with depth of invasion and different metastatic pathways in early stage gastric carcinoma. Cancer. 2000;89:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | Murnane MJ, Sheahan K, Ozdemirli M, Shuja S. Stage-specific increases in cathepsin B messenger RNA content in human colorectal carcinoma. Cancer Res. 1991;51:1137-1142. [PubMed] |
| 53. | Rempel SA, Rosenblum ML, Mikkelsen T, Yan PS, Ellis KD, Golembieski WA, Sameni M, Rozhin J, Ziegler G, Sloane BF. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994;54:6027-6031. [PubMed] |
| 54. | Shuja S, Murnane MJ. Marked increases in cathepsin B and L activities distinguish papillary carcinoma of the thyroid from normal thyroid or thyroid with non-neoplastic disease. Int J Cancer. 1996;66:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Sinha AA, Gleason DF, Deleon OF, Wilson MJ, Sloane BF. Localization of a biotinylated cathepsin B oligonucleotide probe in human prostate including invasive cells and invasive edges by in situ hybridization. Anat Rec. 1993;235:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Hughes SJ, Glover TW, Zhu XX, Kuick R, Thoraval D, Orringer MB, Beer DG, Hanash S. A novel amplicon at 8p22-23 results in overexpression of cathepsin B in esophageal adenocarcinoma. Proc Natl Acad Sci USA. 1998;95:12410-12415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Hazen LG, Bleeker FE, Lauritzen B, Bahns S, Song J, Jonker A, Van Driel BE, Lyon H, Hansen U, Köhler A. Comparative localization of cathepsin B protein and activity in colorectal cancer. J Histochem Cytochem. 2000;48:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Berquin IM, Sloane BF. Cathepsin B expression in human tumors. Adv Exp Med Biol. 1996;389:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Nishimura Y, Amano J, Sato H, Tsuji H, Kato K. Biosynthesis of lysosomal cathepsins B and H in cultured rat hepatocytes. Arch Biochem Biophys. 1988;262:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Lah TT, Cercek M, Blejec A, Kos J, Gorodetsky E, Somers R, Daskal I. Cathepsin B, a prognostic indicator in lymph node-negative breast carcinoma patients: comparison with cathepsin D, cathepsin L, and other clinical indicators. Clin Cancer Res. 2000;6:578-584. [PubMed] |
| 61. | Chabowski A, Skrzydlewska E, Sulkowska M, Famulski W, Sulkowski S, Chrzanowska A. The activity of cathepsin B in colorectal adenocarcinomas. Pol Merkur Lekarski. 2001;11:330-333. [PubMed] |
| 62. | Heidtmann HH, Salge U, Abrahamson M, Bencina M, Kastelic L, Kopitar-Jerala N, Turk V, Lah TT. Cathepsin B and cysteine proteinase inhibitors in human lung cancer cell lines. Clin Exp Metastasis. 1997;15:368-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Maciewicz RA, Wardale RJ, Etherington DJ, Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989;43:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Ohsawa T, Higashi T, Tsuji T. The secretion of high molecular weight cathepsin B from cultured human liver cancers. Acta Med Okayama. 1989;43:9-15. [PubMed] |
| 65. | Sloane BF, Moin K, Sameni M, Tait LR, Rozhin J, Ziegler G. Membrane association of cathepsin B can be induced by transfection of human breast epithelial cells with c-Ha-ras oncogene. J Cell Sci. 1994;107:373-384. [PubMed] |
| 66. | Warwas M, Haczyńska H, Gerber J, Nowak M. Cathepsin B-like activity as a serum tumour marker in ovarian carcinoma. Eur J Clin Chem Clin Biochem. 1997;35:301-304. [PubMed] |
| 67. | Miyake H, Hara I, Eto H. Serum level of cathepsin B and its density in men with prostate cancer as novel markers of disease progression. Anticancer Res. 2004;24:2573-2577. [PubMed] |
| 68. | Leto G, Tumminello FM, Pizzolanti G, Montalto G, Soresi M, Gebbia N. Lysosomal cathepsins B and L and Stefin A blood levels in patients with hepatocellular carcinoma and/or liver cirrhosis: potential clinical implications. Oncology. 1997;54:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Lüthgens K, Ebert W, Trefz G, Gabrijelcic D, Turk V, Lah T. Cathepsin B and cysteine proteinase inhibitors in bronchoalveolar lavage fluid of lung cancer patients. Cancer Detect Prev. 1993;17:387-397. [PubMed] |
| 70. | Nagai A, Terashima M, Harada T, Shimode K, Takeuchi H, Murakawa Y, Nagasaki M, Nakano A, Kobayashi S. Cathepsin B and H activities and cystatin C concentrations in cerebrospinal fluid from patients with leptomeningeal metastasis. Clin Chim Acta. 2003;329:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Buck MR, Karustis DG, Day NA, Honn KV, Sloane BF. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem J. 1992;282:273-278. [PubMed] |
| 72. | Guinec N, Pagano M, Dalet-Fumeron V, Engler R. "In vitro" digestion of intact bovine lens capsules by four human lysosomal cysteine-proteinases. Biol Chem Hoppe Seyler. 1990;371 Suppl:239-254. [PubMed] |
| 73. | Ugarova TP, Ljubimov AV, Deng L, Plow EF. Proteolysis regulates exposure of the IIICS-1 adhesive sequence in plasma fibronectin. Biochemistry. 1996;35:10913-10921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Murphy G, Stanton H, Cowell S, Butler G, Knäuper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 320] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 75. | Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151-160. [PubMed] |
| 76. | Eeckhout Y, Vaes G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem J. 1977;166:21-31. [PubMed] |
| 77. | Murphy G, Ward R, Gavrilovic J, Atkinson S. Physiological mechanisms for metalloproteinase activation. Matrix Suppl. 1992;1:224-230. [PubMed] |
| 78. | Kostoulas G, Lang A, Nagase H, Baici A. Stimulation of angiogenesis through cathepsin B inactivation of the tissue inhibitors of matrix metalloproteinases. FEBS Lett. 1999;455:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Poltorak Z, Cohen T, Sivan R, Kandelis Y, Spira G, Vlodavsky I, Keshet E, Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997;272:7151-7158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 80. | Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51-59. [PubMed] |
| 81. | Arora S, Chauhan SS. Identification and characterization of a novel human cathepsin L splice variant. Gene. 2002;293:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Kirschke H. In: Ansorge S, Langner J, editors, Cellular peptidases in immune functions and diseases, Lysosomal cysteine peptidases and malignant tumours, Plenum Press, New York, London 1997: 253–257. . |
| 83. | Ishidoh K, Saido TC, Kawashima S, Hirose M, Watanabe S, Sato N, Kominami E. Multiple processing of procathepsin L to cathepsin L in vivo. Biochem Biophys Res Commun. 1998;252:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Kirschke H, Eerola R, Hopsu-Havu VK, Brömme D, Vuorio E. Antisense RNA inhibition of cathepsin L expression reduces tumorigenicity of malignant cells. Eur J Cancer. 2000;36:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629-4633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 559] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 86. | Kos J, Stabuc B, Schweiger A, Krasovec M, Cimerman N, Kopitar-Jerala N, Vrhovec I. Cathepsins B, H, and L and their inhibitors stefin A and cystatin C in sera of melanoma patients. Clin Cancer Res. 1997;3:1815-1822. [PubMed] |
| 87. | Popova AN, Zhloba AA, Solov'ev MM. Activity of cathepsin H in blood and biopsies of patients with precancerous conditions and mouth cancer. Vopr Onkol. 2001;47:590-594. [PubMed] |
| 88. | Schweiger A, Staib A, Werle B, Krasovec M, Lah TT, Ebert W, Turk V, Kos J. Cysteine proteinase cathepsin H in tumours and sera of lung cancer patients: relation to prognosis and cigarette smoking. Br J Cancer. 2000;82:782-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Sivaparvathi M, Sawaya R, Gokaslan ZL, Chintala SK, Rao JS. Expression and the role of cathepsin H in human glioma progression and invasion. Cancer Lett. 1996;104:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Waghray A, Keppler D, Sloane BF, Schuger L, Chen YQ. Analysis of a truncated form of cathepsin H in human prostate tumor cells. J Biol Chem. 2002;277:11533-11538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Lah TT, Kos J. Cysteine proteinases in cancer progression and their clinical relevance for prognosis. Biol Chem. 1998;379:125-130. [PubMed] |
| 92. | Tsushima H, Ueki A, Matsuoka Y, Mihara H, Hopsu-Havu VK. Characterization of a cathepsin-H-like enzyme from a human melanoma cell line. Int J Cancer. 1991;48:726-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, Foged NT, Delmas PD, Delaissé JM. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347-32352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 470] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 94. | McQueney MS, Amegadzie BY, D'Alessio K, Hanning CR, McLaughlin MM, McNulty D, Carr SA, Ijames C, Kurdyla J, Jones CS. Autocatalytic activation of human cathepsin K. J Biol Chem. 1997;272:13955-13960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Ishikawa T, Kamiyama M, Tani-Ishii N, Suzuki H, Ichikawa Y, Hamaguchi Y, Momiyama N, Shimada H. Inhibition of osteoclast differentiation and bone resorption by cathepsin K antisense oligonucleotides. Mol Carcinog. 2001;32:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Henskens YM, Veerman EC, Nieuw Amerongen AV. Cystatins in health and disease. Biol Chem Hoppe Seyler. 1996;377:71-86. [PubMed] |
| 98. | Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem. 1997;378:141-150. [PubMed] |
| 99. | Ni J, Abrahamson M, Zhang M, Fernandez MA, Grubb A, Su J, Yu GL, Li Y, Parmelee D, Xing L. Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. J Biol Chem. 1997;272:10853-10858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 100. | Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195-19203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 101. | Levicar N, Kos J, Blejec A, Golouh R, Vrhovec I, Frkovic-Grazio S, Lah TT. Comparison of potential biological markers cathepsin B, cathepsin L, stefin A and stefin B with urokinase and plasminogen activator inhibitor-1 and clinicopathological data of breast carcinoma patients. Cancer Detect Prev. 2002;26:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Zajc I, Sever N, Bervar A, Lah TT. Expression of cysteine peptidase cathepsin L and its inhibitors stefins A and B in relation to tumorigenicity of breast cancer cell lines. Cancer Lett. 2002;187:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Schick C, Pemberton PA, Shi GP, Kamachi Y, Cataltepe S, Bartuski AJ, Gornstein ER, Brömme D, Chapman HA, Silverman GA. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry. 1998;37:5258-5266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 214] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 104. | Bervar A, Zajc I, Sever N, Katunuma N, Sloane BF, Lah TT. Invasiveness of transformed human breast epithelial cell lines is related to cathepsin B and inhibited by cysteine proteinase inhibitors. Biol Chem. 2003;384:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 105. | Boike G, Lah T, Sloane BF, Rozhin J, Honn K, Guirguis R, Stracke ML, Liotta LA, Schiffmann E. A possible role for cysteine proteinase and its inhibitors in motility of malignant melanoma and other tumour cells. Melanoma Res. 1992;1:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Krepela E, Procházka J, Kárová B. Regulation of cathepsin B activity by cysteine and related thiols. Biol Chem. 1999;380:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 107. | Dehrmann FM, Coetzer TH, Pike RN, Dennison C. Mature cathepsin L is substantially active in the ionic milieu of the extracellular medium. Arch Biochem Biophys. 1995;324:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Li Z, Hou WS, Brömme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 109. | Turk B, Bieth JG, Björk I, Dolenc I, Turk D, Cimerman N, Kos J, Colic A, Stoka V, Turk V. Regulation of the activity of lysosomal cysteine proteinases by pH-induced inactivation and/or endogenous protein inhibitors, cystatins. Biol Chem Hoppe Seyler. 1995;376:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Almeida PC, Nantes IL, Chagas JR, Rizzi CC, Faljoni-Alario A, Carmona E, Juliano L, Nader HB, Tersariol IL. Cathepsin B activity regulation. Heparin-like glycosaminogylcans protect human cathepsin B from alkaline pH-induced inactivation. J Biol Chem. 2001;276:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 111. | Edlund T, Ny T, Rånby M, Hedén LO, Palm G, Holmgren E, Josephson S. Isolation of cDNA sequences coding for a part of human tissue plasminogen activator. Proc Natl Acad Sci USA. 1983;80:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 112. | Danø K, Rømer J, Nielsen BS, Bjørn S, Pyke C, Rygaard J, Lund LR. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types. APMIS. 1999;107:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 212] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 113. | Behrendt N, Rønne E, Danø K. The structure and function of the urokinase receptor, a membrane protein governing plasminogen activation on the cell surface. Biol Chem Hoppe Seyler. 1995;376:269-279. [PubMed] |
| 114. | Stephens RW, Pöllänen J, Tapiovaara H, Leung KC, Sim PS, Salonen EM, Rønne E, Behrendt N, Danø K, Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989;108:1987-1995. [PubMed] |
| 115. | Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 116. | España F, Estellés A, Fernández PJ, Gilabert J, Sánchez-Cuenca J, Griffin JH. Evidence for the regulation of urokinase and tissue type plasminogen activators by the serpin, protein C inhibitor, in semen and blood plasma. Thromb Haemost. 1993;70:989-994. [PubMed] |
| 118. | Danø K, Andreasen PA, Grøndahl-Hansen J, Kristensen P, Nielsen LS, Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1740] [Cited by in RCA: 1826] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 119. | Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J. Proteolytic control of growth factor availability. APMIS. 1999;107:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 120. | Hollas W, Blasi F, Boyd D. Role of the urokinase receptor in facilitating extracellular matrix invasion by cultured colon cancer. Cancer Res. 1991;51:3690-3695. [PubMed] |
| 121. | Têtu B, Brisson J, Lapointe H, Bernard P. Prognostic significance of stromelysin 3, gelatinase A, and urokinase expression in breast cancer. Hum Pathol. 1998;29:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 122. | Johansson N, Ala-aho R, Uitto V, Grénman R, Fusenig NE, López-Otín C, Kähäri VM. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113 Pt 2:227-235. [PubMed] |
| 123. | Kanayama H. Matrix metalloproteinases and bladder cancer. J Med Invest. 2001;48:31-43. [PubMed] |
| 124. | Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1868] [Cited by in RCA: 1913] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 125. | Seiki M. Membrane-type matrix metalloproteinases. APMIS. 1999;107:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 209] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 126. | Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197-250. [PubMed] |
| 127. | Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 128. | Fini ME, Cook JR, Mohan R, Brinckerhoff CE. Regulation of matrix metalloproteinases gene expression. Matrix metalloproteinases. San diego (CA): Academic Press 1998; 300-356. |
| 129. | Duivenvoorden WC, Hirte HW, Singh G. Transforming growth factor beta1 acts as an inducer of matrix metalloproteinase expression and activity in human bone-metastasizing cancer cells. Clin Exp Metastasis. 1999;17:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Ellerbroek SM, Hudson LG, Stack MS. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int J Cancer. 1998;78:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 131. | Janowska-Wieczorek A, Marquez LA, Nabholtz JM, Cabuhat ML, Montaño J, Chang H, Rozmus J, Russell JA, Edwards DR, Turner AR. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34(+) cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379-3390. [PubMed] |
| 132. | Kondapaka SB, Fridman R, Reddy KB. Epidermal growth factor and amphiregulin up-regulate matrix metalloproteinase-9 (MMP-9) in human breast cancer cells. Int J Cancer. 1997;70:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 133. | Kurogi T, Nabeshima K, Kataoka H, Okada Y, Koono M. Stimulation of gelatinase B and tissue inhibitors of metalloproteinase (TIMP) production in co-culture of human osteosarcoma cells and human fibroblasts: gelatinase B production was stimulated via up-regulation of fibroblast growth factor (FGF) receptor. Int J Cancer. 1996;66:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 134. | Mira E, Mañes S, Lacalle RA, Márquez G, Martínez-A C. Insulin-like growth factor I-triggered cell migration and invasion are mediated by matrix metalloproteinase-9. Endocrinology. 1999;140:1657-1664. [PubMed] |
| 135. | Sundareshan P, Nagle RB, Bowden GT. EGF induces the expression of matrilysin in the human prostate adenocarcinoma cell line, LNCaP. Prostate. 1999;40:159-166. [PubMed] [DOI] [Full Text] |
| 136. | Reddy KB, Krueger JS, Kondapaka SB, Diglio CA. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int J Cancer. 1999;82:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 137. | Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781-792. [PubMed] |
| 138. | Sato H, Okada Y, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in cell invasion. Thromb Haemost. 1997;78:497-500. [PubMed] |
| 139. | Wernert N, Gilles F, Fafeur V, Bouali F, Raes MB, Pyke C, Dupressoir T, Seitz G, Vandenbunder B, Stéhelin D. Stromal expression of c-Ets1 transcription factor correlates with tumor invasion. Cancer Res. 1994;54:5683-5688. [PubMed] |
| 140. | Karin M, Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2107] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 141. | Reunanen N, Li SP, Ahonen M, Foschi M, Han J, Kähäri VM. Activation of p38 alpha MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360-32368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 142. | Oka H, Chatani Y, Hoshino R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995;55:4182-4187. [PubMed] |
| 143. | Sivaraman VS, Wang H, Nuovo GJ, Malbon CC. Hyperexpression of mitogen-activated protein kinase in human breast cancer. J Clin Invest. 1997;99:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 340] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 144. | Davidson B, Goldberg I, Liokumovich P, Kopolovic J, Gotlieb WH, Lerner-Geva L, Reder I, Ben-Baruch G, Reich R. Expression of metalloproteinases and their inhibitors in adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 1998;17:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 145. | González-Avila G, Iturria C, Vadillo F, Terán L, Selman M, Pérez-Tamayo R. 72-kD (MMP-2) and 92-kD (MMP-9) type IV collagenase production and activity in different histologic types of lung cancer cells. Pathobiology. 1998;66:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 146. | Hashimoto K, Kihira Y, Matuo Y, Usui T. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J Urol. 1998;160:1872-1876. [PubMed] |
| 147. | Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun HJ, Ringert RH. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol. 1998;160:1914-1918. [PubMed] |
| 148. | Sutinen M, Kainulainen T, Hurskainen T, Vesterlund E, Alexander JP, Overall CM, Sorsa T, Salo T. Expression of matrix metalloproteinases (MMP-1 and -2) and their inhibitors (TIMP-1, -2 and -3) in oral lichen planus, dysplasia, squamous cell carcinoma and lymph node metastasis. Br J Cancer. 1998;77:2239-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 149. | Fan YZ, Zhang JT, Yang HC, Yang YQ. Expression of MMP-2,TIMP-2 protein and the ratio of MMP-2/TIMP-2 in gallbladder carcinoma and their significance. World J Gastroenterol. 2002;8:1138-1143. [PubMed] |
| 150. | Zucker S, Hymowitz M, Conner C, Zarrabi HM, Hurewitz AN, Matrisian L, Boyd D, Nicolson G, Montana S. Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Ann N Y Acad Sci. 1999;878:212-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 151. | Heppner KJ, Matrisian LM, Jensen RA, Rodgers WH. Expression of most matrix metalloproteinase family members in breast cancer represents a tumor-induced host response. Am J Pathol. 1996;149:273-282. [PubMed] |
| 152. | Johansson N, Airola K, Grénman R, Kariniemi AL, Saarialho-Kere U, Kähäri VM. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am J Pathol. 1997;151:499-508. [PubMed] |
| 153. | Uría JA, Ståhle-Bäckdahl M, Seiki M, Fueyo A, López-Otín C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997;57:4882-4888. [PubMed] |
| 154. | Murray GI, Duncan ME, O'Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 155. | Yamamoto H, Adachi Y, Itoh F, Iku S, Matsuno K, Kusano M, Arimura Y, Endo T, Hinoda Y, Hosokawa M. Association of matrilysin expression with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Cancer Res. 1999;59:3313-3316. [PubMed] |
| 156. | Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 514] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 157. | Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111-122. [PubMed] |
| 158. | Hofmann UB, Westphal JR, Zendman AJ, Becker JC, Ruiter DJ, van Muijen GN. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type 1 matrix metalloproteinase (MT1-MMP) correlate with melanoma progression. J Pathol. 2000;191:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 159. | Imai K, Yokohama Y, Nakanishi I, Ohuchi E, Fujii Y, Nakai N, Okada Y. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995;270:6691-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 160. | Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, Evans S, Ibrahim H, Le QT, Terris DJ. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883-887. [PubMed] |
| 161. | O-charoenrat P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res. 2000;60:1121-1128. [PubMed] |
| 162. | O-charoenrat P, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Differential modulation of proliferation, matrix metalloproteinase expression and invasion of human head and neck squamous carcinoma cells by c-erbB ligands. Clin Exp Metastasis. 1999;17:631-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 163. | Ohashi K, Nemoto T, Nakamura K, Nemori R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88:2201-2209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 164. | Tomita T, Fujii M, Tokumaru Y, Imanishi Y, Kanke M, Yamashita T, Ishiguro R, Kanzaki J, Kameyama K, Otani Y. Granulocyte-macrophage colony-stimulating factor upregulates matrix metalloproteinase-2 (MMP-2) and membrane type-1 MMP (MT1-MMP) in human head and neck cancer cells. Cancer Lett. 2000;156:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331-5338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1200] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 166. | Velasco G, Pendás AM, Fueyo A, Knäuper V, Murphy G, López-Otín C. Cloning and characterization of human MMP-23, a new matrix metalloproteinase predominantly expressed in reproductive tissues and lacking conserved domains in other family members. J Biol Chem. 1999;274:4570-4576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 167. | Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1163] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 168. | Hofmann UT, Brocker EB, Becker JC. Matrix metallopro-teinases and tumor cell invasion. Res Adv in Cancer. 2002;2:231-240. |
| 169. | Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 533] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 170. | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1340] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 171. | Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2922] [Cited by in RCA: 2915] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 172. | Apte SS, Olsen BR, Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family. J Biol Chem. 1995;270:14313-14318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 216] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 173. | Douglas DA, Shi YE, Sang QA. Computational sequence analysis of the tissue inhibitor of metalloproteinase family. J Protein Chem. 1997;16:237-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 174. | Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375-30380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 389] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 175. | Knäuper V, López-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544-1550. [PubMed] |
| 176. | Henriet P, Blavier L, Declerck YA. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS. 1999;107:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 177. | Krüger A, Sanchez-Sweatman OH, Martin DC, Fata JE, Ho AT, Orr FW, Rüther U, Khokha R. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998;16:2419-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 178. | Koop S, Khokha R, Schmidt EE, MacDonald IC, Morris VL, Chambers AF, Groom AC. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. 1994;54:4791-4797. [PubMed] |
| 179. | Bramhall SR, Neoptolemos JP, Stamp GW, Lemoine NR. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 180. | Karameris A, Panagou P, Tsilalis T, Bouros D. Association of expression of metalloproteinases and their inhibitors with the metastatic potential of squamous-cell lung carcinomas. A molecular and immunohistochemical study. Am J Respir Crit Care Med. 1997;156:1930-1936. [PubMed] |
| 181. | McCarthy K, Maguire T, McGreal G, McDermott E, O'Higgins N, Duffy MJ. High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer. 1999;84:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 182. | Powe DG, Brough JL, Carter GI, Bailey EM, Stetler-Stevenson WG, Turner DR, Hewitt RE. TIMP-3 mRNA expression is regionally increased in moderately and poorly differentiated colorectal adenocarcinoma. Br J Cancer. 1997;75:1678-1683. [PubMed] |
| 183. | Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 277] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 184. | Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 194] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 185. | Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 186. | Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int Immunopharmacol. 2001;1:1651-1667. [PubMed] |
| 187. | Henson PM, Hnson JE, Fitlschen C, Kimani G, Bratton DL, Riches DWH. In: Gallin Ji, Goldstein IM, Snyderman R, editors, Inflammation: Basic principles and clinical correlates, Phagocytic cells: Degranulation and secretion, Raven Press New York 1988: 363-380. . |
| 188. | Watorek W, Farley D, Salvesen G, Travis J. Neutrophil elastase and cathepsin G: structure, function, and biological control. Adv Exp Med Biol. 1988;240:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 189. | Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1471] [Cited by in RCA: 1455] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 190. | Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 340] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 191. | Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3068] [Cited by in RCA: 2977] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 192. | Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 702] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 194. | Jones RD, Hancock JT, Morice AH. NADPH oxidase: a universal oxygen sensor? Free Radic Biol Med. 2000;29:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 195. | Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007-3017. [PubMed] |
| 196. | Das U. A radical approach to cancer. Med Sci Monit. 2002;8:RA79-RA92. [PubMed] |
| 197. | Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 507] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 198. | Zieba M, Suwalski M, Kwiatkowska S, Piasecka G, Grzelewska-Rzymowska I, Stolarek R, Nowak D. Comparison of hydrogen peroxide generation and the content of lipid peroxidation products in lung cancer tissue and pulmonary parenchyma. Respir Med. 2000;94:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 199. | Park JY, Shigenaga MK, Ames BN. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA. 1996;93:2322-2327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 200. | Beinert T, Binder D, Oehm C, Ziemer S, Priem F, Stuschke M, Schweigert M, Siebert G, Mergenthaler HG, Schmid P. Further evidence for oxidant-induced vascular endothelial growth factor up-regulation in the bronchoalveolar lavage fluid of lung cancer patients undergoing radio-chemotherapy. J Cancer Res Clin Oncol. 2000;126:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 201. | Mantovani G, Macciò A, Madeddu C, Mura L, Massa E, Gramignano G, Lusso MR, Murgia V, Camboni P, Ferreli L. Reactive oxygen species, antioxidant mechanisms, and serum cytokine levels in cancer patients: impact of an antioxidant treatment. J Environ Pathol Toxicol Oncol. 2003;22:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 202. | Chung YW, Jeong DW, Won JY, Choi EJ, Choi YH, Kim IY. H(2)O(2)-induced AP-1 activation and its effect on p21(WAF1/CIP1)-mediated G2/M arrest in a p53-deficient human lung cancer cell. Biochem Biophys Res Commun. 2002;293:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 203. | Hsu TC, Young MR, Cmarik J, Colburn NH. Activator protein 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent transcriptional events in carcinogenesis. Free Radic Biol Med. 2000;28:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 204. | Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS. Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem. 1998;273:15846-15853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 205. | Hironaka K, Factor VM, Calvisi DF, Conner EA, Thorgeirsson SS. Dysregulation of DNA repair pathways in a transforming growth factor alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. Lab Invest. 2003;83:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 206. | Höcker M, Rosenberg I, Xavier R, Henihan RJ, Wiedenmann B, Rosewicz S, Podolsky DK, Wang TC. Oxidative stress activates the human histidine decarboxylase promoter in AGS gastric cancer cells. J Biol Chem. 1998;273:23046-23054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 207. | Saari H, Suomalainen K, Lindy O, Konttinen YT, Sorsa T. Activation of latent human neutrophil collagenase by reactive oxygen species and serine proteases. Biochem Biophys Res Commun. 1990;171:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 208. | Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 209. | Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press, Inc. New York, 1999. . |
| 210. | Davies KJ. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987;262:9895-9901. [PubMed] |
| 211. | Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1329] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 212. | Simpson JA, Gieseg SP, Dean RT. Free radical and enzymatic mechanisms for the generation of protein bound reducing moieties. Biochim Biophys Acta. 1993;1156:190-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 213. | Wolff SP, Dean RT. Fragmentation of proteins by free radicals and its effect on their susceptibility to enzymic hydrolysis. Biochem J. 1986;234:399-403. [PubMed] |
| 214. | Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987;262:9902-9907. [PubMed] |
| 215. | Esterbauer H, Gebicki J, Puhl H, Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1706] [Cited by in RCA: 1603] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 216. | Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4998] [Cited by in RCA: 5094] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 217. | de Cavanagh EM, Honegger AE, Hofer E, Bordenave RH, Bullorsky EO, Chasseing NA, Fraga C. Higher oxidation and lower antioxidant levels in peripheral blood plasma and bone marrow plasma from advanced cancer patients. Cancer. 2002;94:3247-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 218. | Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 219. | Travis J. Structure, function, and control of neutrophil proteinases. Am J Med. 1988;84:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 220. | Horton AA, Fairhurst S. Lipid peroxidation and mechanisms of toxicity. Crit Rev Toxicol. 1987;18:27-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 219] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 221. | Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J Cell Biol. 1995;130:157-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 456] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 222. | Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819-1828. [PubMed] |
| 223. | Wefers H, Sies H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem. 1988;174:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 234] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 224. | Fryer MJ. Evidence for the photoprotective effects of vitamin E. Photochem Photobiol. 1993;58:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 225. | Erin AN, Spirin MM, Tabidze LV, Kagan VE. Formation of alpha-tocopherol complexes with fatty acids. A hypothetical mechanism of stabilization of biomembranes by vitamin E. Biochim Biophys Acta. 1984;774:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 226. | Palan PR, Mikhail MS, Shaban DW, Romney SL. Plasma concentrations of coenzyme Q10 and tocopherols in cervical intraepithelial neoplasia and cervical cancer. Eur J Cancer Prev. 2003;12:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 227. | Gumpricht E, Hildenbrandt GR, Scholz RW, Reddy CC. Glutathione-dependent protection against lipid peroxidation in sheep liver microsomes. Biochem Mol Biol Int. 1996;38:559-567. [PubMed] |
| 228. | McCay PB, Brueggemann G, Lai EK, Powell SR. Evidence that alpha-tocopherol functions cyclically to quench free radicals in hepatic microsomes. Requirement for glutathione and a heat-labile factor. Ann N Y Acad Sci. 1989;570:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 229. | Scholz RW, Graham KS, Reddy CC. Glutathione disulfide enhances the reduced glutathione inhibition of lipid peroxidation in rat liver microsomes. Biochem Biophys Res Commun. 1990;166:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 230. | Richter C. Pro-oxidants and mitochondrial Ca2+: their relationship to apoptosis and oncogenesis. FEBS Lett. 1993;325:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 231. | Brunk UT, Zhang H, Dalen H, Ollinger K. Exposure of cells to nonlethal concentrations of hydrogen peroxide induces degeneration-repair mechanisms involving lysosomal destabilization. Free Radic Biol Med. 1995;19:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 232. | Brunk UT, Jones CB, Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 197] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 233. | Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 776] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 234. | Brunk UT, Dalen H, Roberg K, Hellquist HB. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic Biol Med. 1997;23:616-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 238] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 235. | Zhang GJ, Yao J. The direct cause of photodamage-induced lysosomal destabilization. Biochim Biophys Acta. 1997;1326:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 236. | Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919-11927. [PubMed] |
| 237. | O'Neil J, Hoppe G, Sayre LM, Hoff HF. Inactivation of cathepsin B by oxidized LDL involves complex formation induced by binding of putative reactive sites exposed at low pH to thiols on the enzyme. Free Radic Biol Med. 1997;23:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 238. | Zhang HJ, Zhao W, Venkataraman S, Robbins ME, Buettner GR, Kregel KC, Oberley LW. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002;277:20919-20926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 239. | Kleinveld HA, Swaak AJ, Hack CE, Koster JF. Interactions between oxygen free radicals and proteins. Implications for rheumatoid arthritis. An overview. Scand J Rheumatol. 1989;18:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 240. | Kanoh Y, Ohtani N, Mashiko T, Ohtani S, Nishikawa T, Egawa S, Baba S, Ohtani H. Levels of alpha 2 macroglobulin can predict bone metastases in prostate cancer. Anticancer Res. 2001;21:551-556. [PubMed] |
| 241. | Yang P, Cunningham JM, Halling KC, Lesnick TG, Burgart LJ, Wiegert EM, Christensen ER, Lindor NM, Katzmann JA, Thibodeau SN. Higher risk of mismatch repair-deficient colorectal cancer in alpha(1)-antitrypsin deficiency carriers and cigarette smokers. Mol Genet Metab. 2000;71:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 242. | Yang P, Wentzlaff KA, Katzmann JA, Marks RS, Allen MS, Lesnick TG, Lindor NM, Myers JL, Wiegert E, Midthun DE. Alpha1-antitrypsin deficiency allele carriers among lung cancer patients. Cancer Epidemiol Biomarkers Prev. 1999;8:461-465. [PubMed] |
| 243. | Foekens JA, Ries C, Look MP, Gippner-Steppert C, Klijn JG, Jochum M. The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Res. 2003;63:337-341. [PubMed] |
| 244. | Bucurenci N, Blake DR, Chidwick K, Winyard PG. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett. 1992;300:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 245. | Kitagawa S, Takaku F, Sakamoto S. Serine protease inhibitors inhibit superoxide production by human polymorphonuclear leukocytes and monocytes stimulated by various surface active agents. FEBS Lett. 1979;107:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 246. | Dean RT, Nick HP, Schnebli HP. Free radicals inactivate human neutrophil elastase and its inhibitors with comparable efficiency. Biochem Biophys Res Commun. 1989;159:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 247. | Johnson D, Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979;254:4022-4026. [PubMed] |
| 248. | Kramps JA, Willems LN, Franken C, Dijkman JH. Antileukoprotease, its role in the human lung. Biol Chem Hoppe Seyler. 1988;369 Suppl:83-87. [PubMed] |
| 249. | Baker MS, Green SP, Goss N, Katrantzis M, Doe WF. Plasminogen activator inhibitor 2 (PAI-2) is not inactivated by exposure to oxidants which can be released from activated neutrophils. Biochem Biophys Res Commun. 1990;166:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 250. | Steinbrecher UP, Zhang HF, Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 449] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 251. | Carrell RW, Jeppsson JO, Laurell CB, Brennan SO, Owen MC, Vaughan L, Boswell DR. Structure and variation of human alpha 1-antitrypsin. Nature. 1982;298:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 482] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 252. | Travis J, Salvesen GS. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655-709. |
| 253. | Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med. 1995;18:93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 688] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 254. | Naskalski JW, Marcinkiewicz J, Drozdz R. Myeloperoxidase-mediated protein oxidation: its possible biological functions. Clin Chem Lab Med. 2002;40:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 255. | Huber R, Carrell RW. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951-8966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 696] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 256. | Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992;7:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 431] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 257. | Capodici C, Berg RA. Hypochlorous acid (HOCl) activation of neutrophil collagenase requires cathepsin G. Agents Actions. 1989;27:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 258. | Desrochers PE, Weiss SJ. Proteolytic inactivation of alpha-1-proteinase inhibitor by a neutrophil metalloproteinase. J Clin Invest. 1988;81:1646-1650. [PubMed] |
| 259. | Vissers MC, George PM, Bathurst IC, Brennan SO, Winterbourn CC. Cleavage and inactivation of alpha 1-antitrypsin by metalloproteinases released from neutrophils. J Clin Invest. 1988;82:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 260. | Winyard PG, Zhang Z, Chidwick K, Blake DR, Carrell RW, Murphy G. Proteolytic inactivation of human alpha 1 antitrypsin by human stromelysin. FEBS Lett. 1991;279:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 261. | Zhang Z, Winyard PG, Chidwick K, Farrell A, Pemberton P, Carrell RW, Blake DR. Increased proteolytic cleavage of alpha 1-antitrypsin (alpha 1-proteinase inhibitor) in knee-joint synovial fluid from patients with rheumatoid arthritis. Biochem Soc Trans. 1990;18:898-899. [PubMed] |
| 262. | Wong PS, Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980;96:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 263. | Cassiman JJ, van Leuven F, van der Schueren B, van den Berghe H. Immunohistochemical localization of human alpha 2Macroglobulin in connective tissue. Cell Tissue Res. 1980;213:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 264. | Brown PD. Clinical studies with matrix metalloproteinase inhibitors. APMIS. 1999;107:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 265. | Ahonen M, Baker AH, Kähäri VM. Adenovirus-mediated gene delivery of tissue inhibitor of metalloproteinases-3 inhibits invasion and induces apoptosis in melanoma cells. Cancer Res. 1998;58:2310-2315. [PubMed] |
| 266. | Greenberger JS, Kagan VE, Pearce L, Boriseniao G, Tyurina Y, Epperly MW. Modulation of redox signal transduction pathways in the treatment of cancer. Antioxid Redox Signal. 2001;3:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 267. | Hileman EA, Achanta G, Huang P. Superoxide dismutase: an emerging target for cancer therapeutics. Expert Opin Ther Targets. 2001;5:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Assistant Editor Guo SY Edited by Wang XL and Gabbe M