Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1220
Revised: July 8, 2004
Accepted: August 30, 2004
Published online: February 28, 2005
AIM: To investigate the effect of CpG-containing oligodeoxynucleotides (CpG ODN) alone or in combination with the chemotherapeutic agent 5-fluorouracil (5-FU) on tumor growth and whether CpG ODN can reverse the immunosuppression caused by the chemotherapy with 5-FU in murine hepatoma model.
METHODS: Hepatoma model was established by subcutaneous inoculation with hepatoma-22 (H22) cells into the right flank of BALB/c mice. Mice with tumor were treated by peritumoral injection of CpG ODN alone or in combination with subcutaneous injection of 5-FU. Tumor size was quantified regularly. Serum levels of IL-12 and IFN-γ in mice were assayed by enzyme-linked immunosorbent assay (ELISA). The lytic capacity of splenic NK cells was tested by lactate dehydrogenase release assay.
RESULTS: Peritumoral injection of CpG ODN alone or in combination with subcutaneous injection of 5-FU, and the treatment with 5-FU alone all led to significant inhibition of hepatoma growth. The mean tumor volumes fell by 46.66% in mice injected with CpG ODN, 68.34% in the 5-FU treated mice, and 70.23% in mice treated with the combination of CpG ODN and 5-FU than in controls. There was no significant difference in tumor size between 5-FU-treated mice and mice treated with the combination of 5-FU and CpG ODN (P>0.05). The serum levels of IL-12 and IFN-γ of mice treated with CpG ODN alone (IL-12: 464.50±24.37 pg/mL; IFN-γ: 134.20±25.76 pg/mL) or with the co-administration of CpG ODN and 5-FU (IL-12: 335.83±28.74 pg/mL; IFN-γ: 111.00±5.33 pg/mL) were significantly higher than that of controls (IL-12: 237.50±45.31 pg/mL; IFN-γ: 56.75±8.22 pg/mL). The production of IL-12 and IFN-γ was suppressed moderately in 5-FU-treated mice (IL-12: 166.67±53.22 pg/mL; 53.33±16.98 pg/mL) compared to control mice (P>0.05), whereas the combination of CpG ODN and 5-FU significantly increased the serum levels of IL-12 and IFN-γ compared to 5-FU alone (P<0.05). The NK cell killing activity in CpG ODN-treated mice (44.04±1.38%) or the mice treated with CpG ODN combined with 5-FU (30.67±1.28%) was significantly potentiated compared to controls (19.22±0.95%, P<0.05). The co-administration of CpG ODN and 5-FU also significantly enhanced the lytic activity of NK cells when compared with the treatment with 5-FU alone (12.03±1.42%, P<0.05).
CONCLUSION: The present data suggests that CpG ODN used as single therapeutic agent triggers anti-tumor immune response to inhibit the growth of implanted hepatoma and reverses the immunosuppression caused by the chemotherapy with 5-FU.
- Citation: Wang XS, Sheng Z, Ruan YB, Guang Y, Yang ML. CpG oligodeoxynucleotides inhibit tumor growth and reverse the immunosuppression caused by the therapy with 5-fluorouracil in murine hepatoma. World J Gastroenterol 2005; 11(8): 1220-1224
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1220.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1220
CpG is a dinucleotide containing cytosine (C) and guanine (G). Bacterial DNA and synthetic oligodeoxynucleotides containing the CpG motifs (CpG ODN) have been shown to be potent activators of immune system, which can provoke various immune cells and induce the production of a wide variety of T helper-1 (Th1)-promoting cytokines, such as interleukin 12 (IL-12), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and IL-6[1-4]. As a strong immunostimulatory agent, CpG ODN is able to prevent effectively against bacterial, viral and parasitic infections[5-7]. CpG ODN also enhances the anti-tumor efficacy of monoclonal antibodies or cancer vaccines when used as immune adjuvants in animal tumor models[8,9]. The administration of CpG ODN alone is also capable of triggering potent anti-tumor immune responses against various experimental tumors, for example, lymphoma, leukemia, melanoma, colon tumor, glioma, cervical cancer, neuroblastoma[8,10-14].
Among the available approaches used for the treatment of hepatocellular carcinoma (HCC), chemotherapy is still a major alternative for the majority of cases. However, a single chemotherapeutic agent usually was shown to be poor in suppressing HCC and improving the overall survival rate of patients with HCC[15]. Immunotherapy, as a novel option for tumor management, has offered some clinical benefits for improvement of the survival patients with HCC[16]. In particular, the combination therapy with chemotherapeutic agents and immunostimulators, such as 5-fluorouracil (5-FU) and IFN, has been found to be effective in enhancing the HCC-inhibitory effect of chemotherapy alone[16,17]. In the present study, we investigated the therapeutic potential of CpG ODN by peritumoral injection in murine hepatoma model and the possibility that the combination CpG ODN with 5-FU promotes the inhibitory efficacy of 5-FU alone.
The production of large amounts of IL-12 and IFN-γ, and the activation of natural killer (NK) cells has been thought to be an important property of the CpG ODN-elicited immune response[5-7,18]. In this study, we evaluated the effects of CpG ODN on immune cells by examining the serum levels of IL-12 and IFN-γ and cytotoxicity of splenic NK cells in mice with tumor to further understand the possible mechanism responsible for CpG ODN action. The chemotherapeutic drug 5-FU usually causes severe bone marrow suppression leading to immunosuppression; in contrast, CpG ODN is a potent immune activator; hence we also explored whether or not CpG ODN used in combination with 5-FU could improve the immunosuppressive condition in 5-FU-treated mice in terms of IL-12 and IFN-γ contents in murine sera and NK cell killing activity.
BALB/c mice were obtained from the Animal Center of Tongji Medical College (Wuhan, China) and used for studies at ages of 6 to 8 wk with an average body weight of 20 g.
Mouse hepatoma cell line, hepatoma-22 (H22) and murine lymphoma YAC-1 cell line were provided by China Center for Type Culture Collection (Wuhan, China). H22 cells were maintained in our laboratory by injecting intraperitoneally 1×106 cells into the above-mentioned BALB/c mice. YAC-1 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/mL of penicillin, 100 µg/mL of streptomycin and 2 mmol/L L-glutamine at 37 °C, 50 mL/L CO2. All culture reagents were purchased from Gibco BRL, UK.
The ODNs used in the present study included CpG ODN 1826 and the non-CpG control ODN 1982[19]. Both of the purified ODNs were purchased from Shanghai Sangon Biological Engineering Technology Service Co. Ltd. (Shanghai, China). The sequence of CpG ODN 1826 was: 5’-TCCATGACGTTCCTGACGTT-3’, which contains two CpG dinucleotides. The non-CpG ODN 1982 sequence was: 5’-TCCAGGACTTCTCTCAGGTT-3’. Before in vivo use, ODNs were dissolved in sterile isotonic sodium chloride.
H22 cells harvested from the peritoneal cavity of the mice bearing hepatoma-22 were washed three times with cold PBS. 1×106 of cells with a viability of >95%, as determined by trypan blue exclusion, were inoculated subcutaneously at the right hind flank of BALB/c mice. The implanted tumors exhibited 100% survival. When the subcutaneous tumor diameter had reached 4-5 mm (5 d after tumor inoculation), treatment with ODNs alone or in combination with 5-FU was started. Mice were injected peritumorally with 50 µL of sodium chloride per day (control) or 50 µg of ODNs dissolved in 50 µL of saline (once per day) on d 5, 10, 15 and 17 after tumor inoculation. From the first day of treatment, 5-FU (25 mg/kg) dissolved in 50 µL of saline was given at the back of mice per day subcutaneously for 12 consecutive days with or without peritumoral injection of ODN (CpG ODN) as described above. Tumor diameter were measured every third day with a caliper and tumor volume was calculated according to the following formula: V = (largest diameter)×(smallest diameter)2/2. For each experiment, groups of 5-8 mice were used.
Peripheral blood was collected from mice with tumor 24 h after the final injection of ODNs. Sera were separated and frozen at -70 °C. Serum levels of IL-12 (p40) and IFN-γ were measured using enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Inc., CA, USA) according to the manufacturer’s instructions. The results were expressed as pg/mL.
After collection of blood, mice were killed by cervical dislocation. Spleen was freshly removed and smashed in cold RPMI 1640 media; a single-cell suspension was obtained by passing the material through a sterile nylon mesh of 100 and 200 gauge in turn. Lymphocytes were separated from suspension by gradient centrifugation with Ficoll-Hypaque with a specific gravity of 1.09. NK cell activity was determined by the lactate dehydrogenase (LDH) release assay[20]. Briefly, purified lymphocytes were incubated with the NK-sensitive target cell YAC-1 at E:T ratios of 100 for 4 h in triplicates at 37 °C in a 50 mL/L CO2 incubator. The spontaneous and maximum release of LDH was determined by culturing YAC-1 cells in culture medium alone or with 1% sodium lauryl sulfate. After incubation, the enzymatic reactions were carried out in the dark over a period of 15 min according to the methods described by Konjevic et al[20]. All reagents were purchased from Sigma (St. Louis, MO). The absorbances were read at 570 nm and NK cell lytic activity was calculated by the following formula: lytic activity (%) = (experimental release - spontaneous release)/(maximum release - spontaneous release) ×100%.
All results were expressed as mean±SE of individual animal values in each group. Statistical analyses were performed by the unpaired Student’s t test. Results were considered significant at P<0.05.
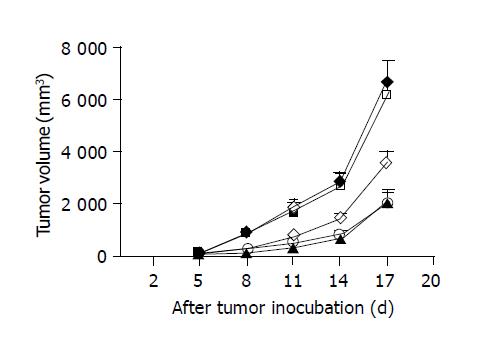
The average survival duration of mice, bearing hepatoma without any treatment was 23 d. Considering that chemotherapeutic or immunostimulatory agents would lose their therapeutic efficacy with the progress of tumor, we killed all mice 18 d after tumor inoculation to compare the effects of different therapeutic agents on tumor growth. Eight days after tumor cell inoculation (3 d after the initial treatments), subcutaneous injection of 5-FU or peritumoral injection of CpG ODNs, but not non-CpG ODN, led to greater reduction of tumor volumes compared to controls injected with sodium chloride (Figure 1). By 17 d of post-tumor implantation, the mean tumor volumes fell by 46.66% in mice injected with CpG ODN (n = 8), which is greater than that seen in controls (n = 5; 3579.38±480.72 vs 6710.40±909.80 mm3; P<0.005). A stronger tumor inhibition occurred in 5-FU-treated mice (n = 8), with tumor volumes 68.34% smaller than controls (2124.56±434.04 vs 6710.40±909.80 mm3, P<0.001). The tumor growth inhibition was not observed in non-CpG ODN-treated mice (6780.70±656.41 mm3, n = 5). The results suggest that the antitumoral effect of CpG ODN was dependent on the CpG motifs within the ODN.
Treatment with the combination of CpG ODN and 5-FU (n = 8) resulted in a 70.23% reduction of mean tumor volumes compared to controls (1997.78±474.56 vs 6710.40±909.80 mm3, P<0.001). There was no significant difference in the tumor inhibition between mice treated with 5-FU alone and combined use of CpG ODN and 5-FU (Figure 1). The result showed that CpG ODN did not produce a cooperative inhibition of hepatoma growth with 5-FU.
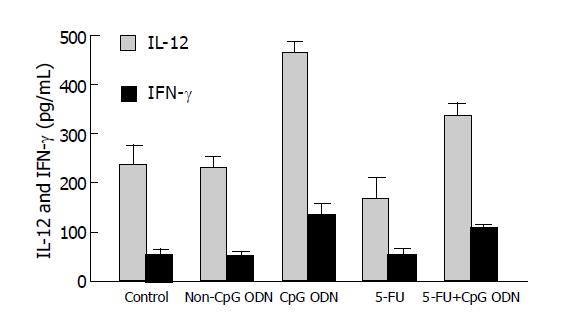
To assess the effects of CpG ODN on immune cell activity in mice with hepatoma, we examined and compared the serum levels of IL-12 and IFN-γ in all mice after different treatments. As shown in Figure 2, concentrations of IL-12 and IFN-γ in sera of mice treated with CpG ODN were significantly higher than those in sera of controls (IL-12: 464.50±24.37 vs 237.50±45.31 pg/mL, P<0.01; IFN-γ: 134.20±25.76 vs 56.75±8.22 pg/mL, P<0.05). There was also a significant increase in serum levels of IL-12 and IFN-γ of mice treated with the co-administration of CpG ODN and 5-FU compared to controls (IL-12: 335.83±28.74 vs 237.50±45.31 pg/mL, P<0.05; IFN-γ: 111.00±5.33 vs 56.75±8.22 pg/mL, P<0.05), whereas non-CpG ODN treatment did not affect the levels of IL-12 and IFN-γ. The results indicated that CpG ODN could induce the production of IL-12 and IFN-γ in mice bearing tumor and the stimulatory effects were dependent on the CpG motifs containing ODN. The production of IL-12 and IFN-γ was suppressed moderately in 5-FU-treated mice compared to control mice, whereas the combination of CpG ODN and 5-FU significantly increased serum levels of IL-12 and IFN-γ compared to 5-FU alone (IL-12: 335.83±28.74 vs 166.67±53.22 pg/mL, P<0.05; IFN-γ: 111.00±5.33 vs 53.33±16.98 pg/mL, P<0.05). The data suggest that CpG ODN can reverse the inhibition of immune cells resulting from the 5-FU therapy.
To evaluate the effect of CpG ODN on the activity of splenic NK cells, we detected the lytic activity of NK cells against YAC1 tumor target cells (Table 1). NK cell killing activity in CpG ODN-treated mice and the mice treated with CpG ODN combined with 5-FU was significantly higher than those in controls, whereas the potentiation of NK cell activity was not seen in non-CpG ODN-treated mice. This observation showed the ability of CpG ODN to activate NK cells and augment their cytotoxicity, and the CpG motifs were necessary for the immunostimulatory activity of CpG ODN. Injection with 5-FU alone caused a relatively mild but no significant inhibition of NK cell compared to control animals. However, the lytic activity of NK cells in mice treated with the combination of CpG ODN and 5-FU is significantly higher than those in 5-FU-treated mice (P<0.05), which suggests that CpG ODN is capable of eliminating the inhibition of NK cell function induced by 5-FU.
CpG ODN 1826 is known as a strong immune activator and induces protective and curative Th1 responses against infections and tumor in vivo, and the immunostimulatory effects of CpG ODN 1826 are dependent on the CpG motifs within oligodeoxynucleotides[5-7,21]. Our studies here first showed that peritumoral injection of CpG ODN 1826 as a single therapeutic agent suppressed the growth of implanted hepatoma in mice, but the oligodeoxynucleotide without CpG motifs was ineffective. The findings are consistent with the previous observations obtained in several tumor models treated with CpG ODN 1826[11,19,21].
The most important immunostimulatory property of CpG ODN is to activate directly antigen-presenting cells (APCs), including macrophages, monocytes and dendritic cells (DCs), to produce a large amount of Th1-promoting cytokines especially IL-12 and IFN-γ[13,14,22]. IL-12 and IFN-γ are critical cytokines in the induction of cellular immunity. IL-12 can promote Th1 cell immune response and stimulate T cells and NK cells to secrete IFN-γ[23]. The activated NK cells also release IL-12, which together with IFN-γ, in turn further activate NK cells, APCs and T cells and enhance their immune activity[18,24]. It has been reported that the IL-12 and IFN-γ-dependent immunological mechanisms are responsible for the CpG ODN-triggered protective response to infections[5-7]. The immunological activity of CpG ODN in the antitumoral response varies with the ODN sequence and backbone as well as tumor models. For example, CpG ODN 1585 was ineffective in lymphoma model but induced a significant regression of tumor in melanoma via NK cells, whereas the inhibition of CpG ODN 1826 in lymphoma model was dependent on both NK and T cells[23]. In the present study, CpG ODN induced production of IL-12 and IFN-γ and potentiated NK cell lytic activity, which exhibited the ability of CpG ODN to activate various immune cells of the mice with hepatoma and to elicit the anti-tumor response. The results also suggest that the production of IL-12 and IFN-γ as well as NK cell activation may play a role in the CpG ODN 1826-induced inhibition of hepatoma growth.
So far, the efficacy of chemotherapeutic agents remains superior to single immune modulators in the management of HCC, which was also confirmed in the present result that 5-FU showed stronger inhibitory effect on hepatoma growth than CpG ODN. Unfortunately, 5-FU inhibits cancer cell growth but also damages normal cells, especially the hemopoietic cells within bone marrow, which can cause bone marrow suppression leading to immunosuppression. CpG ODN is a potent immunostimulator; besides the activation of various immune cells, it has been shown to trigger extramedullary hematopoiesis and enhance cytotoxic T cell function in the irradiated mice[25]. IL-12 derived from activated APCs has been reported to promote maturation of primitive bone marrow precursors and proliferation of DCs synergistically with other hemopoietic growth factors[23]. Theoretically the co-administration of 5-FU and CpG ODN 1826 in mice with hepatoma should be able to reverse the depressed immune function resulting from 5-FU therapy, thereby enhancing the anti-tumor efficacy of 5-FU alone. Our results that the treated mice with CpG ODN combined with 5-FU had higher serum levels of IL-12 and IFN-γ and stronger NK cell lytic activity than the 5-FU-treated mice suggest that CpG ODN indeed reconstitutes immune function of mice treated with 5-FU. However, no significant difference in mean tumor volumes was observed between the two treatments. The results reflected the possibility that the combined use of CpG ODN with 5-FU did not increase the tumor-inhibitory effect of 5-FU. But it is mostly possible that the dose of 5-FU used here is enough to inhibit cancer cells, which cannot be exceeded by the addition of CpG ODN. Even if the combination of CpG ODN with 5-FU failed to produce synergistic inhibition of tumor growth, the immune-potentiating effect induced by CpG ODN undoubtedly contributes to the anti-tumor response in mice, and increases the toleration to the chemotherapeutic agents, thereby bringing about positive effect on tumor control.
In this study, peritumoral injection of CpG ODN did not produce any adverse effects showing that CpG ODN is safer than chemotherapeutic drugs or other immunostimulatory agents such as IL-2 and IFN in tumor treatment. Furthermore, CpG ODN is cheaper and easily available. Therefore, the present findings that CpG ODN inhibited hepatoma growth and triggered an immune response to reverse the immunosuppression caused by 5-FU therapy give rise to the interest that CpG ODN may also be a useful agent in human HCC management.
| 1. | Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2735] [Cited by in RCA: 2632] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 2. | Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042-3049. [PubMed] |
| 3. | Häcker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, Heeg K, Lipford GB, Wagner H. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230-6240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 493] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 288] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, Vogel JC. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970-6975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 171] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Harandi AM, Eriksson K, Holmgren J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J Virol. 2003;77:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect Immun. 2001;69:1643-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Wooldridge JE, Ballas Z, Krieg AM, Weiner GJ. Immunostimulatory oligodeoxynucleotides containing CpG motifs enhance the efficacy of monoclonal antibody therapy of lymphoma. Blood. 1997;89:2994-2998. [PubMed] |
| 9. | Davila E, Celis E. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol. 2000;165:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Blazar BR, Krieg AM, Taylor PA. Synthetic unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides are potent stimulators of antileukemia responses in naive and bone marrow transplant recipients. Blood. 2001;98:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Sharma S, Karakousis CP, Takita H, Shin K, Brooks SP. Intra-tumoral injection of CpG results in the inhibition of tumor growth in murine Colon-26 and B-16 tumors. Biotechnol Lett. 2003;25:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469-2473. [PubMed] |
| 13. | Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9:2693-2700. [PubMed] |
| 14. | Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429-5432. [PubMed] |
| 15. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Leung TW, Patt YZ, Lau WY, Ho SK, Yu SC, Chan AT, Mok TS, Yeo W, Liew CT, Leung NW. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res. 1999;5:1676-1681. [PubMed] |
| 18. | Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840-1845. [PubMed] |
| 19. | Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdörfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Konjević G, Jurisić V, Banićevic B, Spuzić I. The difference in NK-cell activity between patients with non-Hodgkin's lymphomas and Hodgkin's disease. Br J Haematol. 1999;104:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Ballas ZK, Krieg AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, Weiner GJ. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol. 2001;167:4878-4886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 814] [Cited by in RCA: 794] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 23. | Lamont AG, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 157] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 218] [Article Influence: 7.5] [Reference Citation Analysis (0)] |