Published online Feb 28, 2005. doi: 10.3748/wjg.v11.i8.1155
Revised: July 18, 2004
Accepted: August 25, 2004
Published online: February 28, 2005
AIM: To investigate the therapeutic effects of Guiyuanfang and bone marrow stem cells (BMSCs) on rats with liver fibrosis.
METHODS: Liver fibrosis model was induced by carbon tetrachloride, ethanol, high lipid and assessed biochemically and histologically. Liver function and hydroxyproline contents of liver tissue were determined. Serum hyaluronic acid (HA) level and procollagen III level were performed by radioimmunoassay. The VG staining was used to evaluate the collagen deposit in the liver. Immunohistochemical SABC methods were used to detect transplanted BMSCs and expression of urokinase plasminogen activator (uPA).
RESULTS: Serum transaminase level and liver fibrosis in rats were markedly reduced by Guiyuanfang and BMSCs. HA level and procollagen III level were also reduced obviously, compared to model rats (HA: 47.18±10.97 ng/mL, 48.96±14.79 ng/mL; PCIII: 22.48±5.46 ng/mL, 26.90±3.35 ng/mL; P<0.05). Hydroxyproline contents of liver tissue in both BMSCs group and Guiyuanfang group were far lower than that of model group (1227.2±43.1 μg/g liver tissue, 1390.8±156.3 μg/g liver tissue; P<0.01). After treatment fibrosis scores were also reduced. Both Guiyuanfang and BMSCs could increase the expression of uPA. The transplanted BMSCs could engraft, survive, and proliferate in the liver.
CONCLUSION: Guiyuanfang protects against liver fibrosis. Transplanted BMSCs may engraft, survive, and proliferate in the fibrosis livers indefinitely. Guiyuanfang may synergize with BMSCs to improve recovery from liver fibrosis.
- Citation: Wu LM, Li LD, Liu H, Ning KY, Li YK. Effects of Guiyuanfang and autologous transplantation of bone marrow stem cells on rats with liver fibrosis. World J Gastroenterol 2005; 11(8): 1155-1160
- URL: https://www.wjgnet.com/1007-9327/full/v11/i8/1155.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i8.1155
China has a high incidence of hepatic injury and fibrosis. Current therapeutic options for liver fibrosis, unfortunately, remain inadequate and as a result, the social economic burden remains high. Until now, effective drugs are still lacking, so it is most urgent that some novel effective therapies be developed. In near future, targeting of stellate cells, gene therapy, cytokine antagonist, and fibrogenic mediators will be a mainstay of antifibrotic therapy. Natural extracts with putative antifibrotic activity have gained increasing attention[1]. TCM could lead to new prospects for the therapy of fibrosis. A lot of Traditional Chinese Materia Medica, such as Danshen, Danggui, Gancao[2], Xiaocaihu Tang, Fufang 861, Fuzhenghuayufang, and so on, have been documented effective. Our previous study showed that the Guiyuanfang was very effective for liver injury.
Severe liver dysfunction on terminal liver failure can be reversed by orthotopic liver transplantation, but with the necessity for chronic immunosuppression of the recipient and with the need to wait for a donor liver, sometimes for years, due to paucity of liver donors. A variety of technical, financial, and logistic issues, however, limits its application to a relatively small percentage of patients with liver disease.
Hepatocyte transplantation is emerging as a potential treatment to augment cell number in diseased liver, and to show early promising[3,4]. Hepatocyte transplantation is now being done successfully to sustain liver function in patients awaiting liver transplantation[5]. It not only provides temporary liver function in patients but has also shown to be curative in certain metabolic conditions[6,7]. This procedure may be even an alternative to orthotopic liver transplantation. Theoretically, hepatocyte transplantation would be less invasive and technically less demanding than whole organ transplantation and, if the procedure could be performed percutaneously, recipients would not require prolonged hospitalization. Isolated liver cells can be cryopreserved for emergency use[8,9].
The major limitation to this form of therapy is the availability of human livers as a source of hepatocytes. Others were immune allograft rejection and proliferative capacity of liver cells. The bone marrow may be a possible adult reservoir of totipotent stem cells or a novel location for various types of determined stem cells[10-12]. Bone marrow cells can be easily obtained from adult marrow opening the possibility of autologous transplantation. Autologous stem cell transplantation for the treatment of diseased liver is becoming an increasingly promising strategy. We propose to alleviate or overcome the liver problem by developing novel therapies using autologous bone marrow stem cells (BMSCs) and TCM. We attempt to confirm the anti-fibrotic activities of TCM and autologous BMSCs. Could the BMSCs survive, proliferate, and differentiate in the fibrotic environment? Could TCM synergize with the BMSCs to improve the diseased liver?
Normal Sprague-Dawley rats weighing 180-220 g were obtained from Beijing Tonglihua Experimental Animal Center (China). All animals received care in compliance with the guidelines of China Ministry of Health.
The Guiyuanfang consists of four Traditional Chinese Materia Medica purchased from Beijing Tongrentang Pharmaceutical Co., Ltd (Beijing, China), all the herbal was identified by Professor Li JS in the Beijing University of Traditional Chinese Medicine (TCM). ALT Kit, AST Kit, Albumin Kit, and Total Protein kit were all purchased from ZhongShan Biotechnology Co. Ltd (Beijing, China). 5-Bromide-2’-deoxygen-uridine (BrdU) was purchased from Boehringer Co., Ltd (Germany). SABC kit, DSABC kit, and antibody were purchased from Boster Company (Wuhan, China). Hydroxyproline kit was purchased from Jiancheng Biotechnology Co., Ltd (Nanjing, China). All other reagents used were of analytical grade.
Bone marrow cells were collected from the femora of Sprague-Dawley rats. Mononuclear cells were obtained by a Percoll-separation step. The mononuclear cells were inoculated at a density of 1×105/cm2 into 6-well plates. These plates were pre-coated with 0.1% Matrigel (BD). Basal media used were α-MEM supplemented with 10 ng/mL EGF (BD) and 5% fetal bovine serum (Hyclone). EGF was freshly added when the medium was changed every 3 d. Each culture was labeled according to the animal numbers. After 4 wk the cultures were transplanted to the liver of the same rats. Before the transplantation the 5 μL BrdU solution (50 mg BrdU dissolved in 0.8 mL DMSO, added 1.2 mL ddH2O) was added into the cultures.
Fibrosis was induced using carbon tetrachloride, alcohol, and high lipid diet. Briefly, rats were given alcohol (diluted 1:9 in water) added to the drinking water. CCl4 (diluted 4:6 in olive oil) was given subcutaneously twice a week. The initial dose was 0.5 mL/kg body weight, but subsequent dose was adjusted weekly based on the changes in body weight. When there was no change or an increase in body weight, CCl4 was given at 0.3 mL/kg. When body weight decreased by 1-10 g, CCl4 was given at 0.15 mL/kg. When body weight decreased by more than 10 g, CCl4 was not given and the CCl4 dose was reassessed 1 wk later. During the fibrotic model replicated, the rats were fed a high-lipid diet (corn powder 79.5%, lard 20%, 0.5% cholesterol). Eight weeks later, rats were randomly allocated to the different groups. For BMSCs transplantation, a small surgical incision was made in the flank and the portal vein was exposed. Five million BMSCs, suspended in 0.5 mL αMEM medium, were injected into the portal vein in 5 min. At the end of the experiment all rats were killed, blood samples were drawn from celiac artery, and the sera were isolated and stored at -20 °C, a portion of the liver was flash frozen in liquid nitrogen and stored at -70 °C until assayed or fixed in 10% neutral buffered formalin for 18-24 h and paraffin embedded.
Sera were analyzed for alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), and total protein (TP), and hydroxyproline contents of liver tissue were determined by a biochemical kit according to the manufacturer’s instructions. The assay of serum hyaluronic acid (HA) level and procollagen III level was performed with specific radioimmunoassay kit.
Samples were obtained from the same liver lobe in all animals and fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin or Van Gieson solutions. Fibrosis was scored according to the following scoring system modified from HAI System[13]; I = thickened perivenular collagen and a few thin collagen septa; II = thin septa with incomplete bridging between portal regions; III = thin septa and extensive bridging; IV = thickened septa with complete bridging of portal regions and a nodular appearance.
Indirect immunohistochemistry analysis of urokinase plasminogen activators (uPAs) expression was performed on liver sections. Briefly, tissue sections were deparaffinized, and endogenous peroxidase activity was blocked by incubation of tissue in 3% H2O2 for 10 min. Primary anti-uPA antibody was applied at 4 °C for overnight; rabbit IgG was used as a negative control. Then they were incubated with second antibody, biotin-labeled goat anti-rabbit IgG antibody (1:400 dilution), at 37 °C for 2 h, followed by incubation with SABC complex (1:400 dilution), at room temperature for 1 h. Finally, diaminobenzedine was used for color developing, and then the slides were counterstained by hematoxylin.
Dual immunohistochemistry was used to detect the BMSCs in the liver parenchyma and their differentiating state. Monoclonal antibodies against BrdU were used to localize the transplanted bone marrow cells. Briefly, samples were serially rehydrated with 100, 95, and 70% ethanol after deparaffinization with toluene. Endogenous peroxidase in the sample was blocked using 3% hydrogen peroxide for 10 min at room temperature. The sample was treated with pepsin for 5 min at 42 °C and 2 N HCl for 30 min at room temperature. After rinsing with PBS three times, the sample was incubated with antibodies against BrdU in a moist chamber for 16 h at 4 °C. Then they were incubated with second antibody, biotin-labeled goat anti-rabbit IgG antibody (1:400 dilution), at 37 °C for 2 h, followed by incubation with SABC-AP complex (1:400 dilution) at room temperature for 1 h. BCIP/NBT was used for color developing. After rinsing, monoclonal antibodies against cytokeratin-18 were applied to the slides, the other procedures were similar, except that the SABC-POD was used to replace the SABC-AP, and the AEC solutions to replace BCIP/NBT. Negative control samples were incubated in PBS (without the primary antibodies) under similar conditions.
The data are presented as mean±SD. The significance of differences was analyzed with the Student’s t, Mann-Whitney rank sum, χ2 tests or analysis of variance (ANOVA) with the pairwise multiple comparison method of q test using SPSS 10.0 software. P<0.05 was considered significant.
Laboratory measures of liver function remained abnormal in model animals but significantly improved toward normal in animals that received treatment. Administration of Guiyuanfang or BMSC transplantation reduced the elevated serum ALT and AST levels significantly (P<0.01), and serum TP, ALB, A/G levels increased in all animals receiving treatment (P<0.01 or 0.05), compared to model rats (Table 1).
| Group | n | ALT (U/mL) | AST (U/mL) | TP (g/L) | ALB (g/L) | A/G |
| Control | 10 | 38.3±5.1b | 127.9±16.3b | 62.6±5.9 | 28.8±3.5b | 0.85±0.06b |
| Model | 10 | 325.9±101.7 | 697.5±215.8 | 53.2±10.5 | 21.6±3.0 | 0.70±0.09 |
| Guiyuanfang | 10 | 56.8±11.5b | 149.5±31.7b | 64.0±7.0 | 28.3±2.6b | 0.80±0.07b |
| BMSCs | 8 | 67.3±22.0b | 133.3±33.8b | 60.6±8.8 | 26.1±2.2a | 0.75±0.03a |
| Guiyuanfang plus BMSCs | 8 | 40.4±9.5b | 123.1±27.9b | 57.7±5.1 | 26.9±4.2b | 0.83±0.02b |
The serum PCIII, and HA levels were often used as indicators of hepatic fibrosis clinically. As shown in Table 2, the serum PCIII, HA levels of the model rats maintained high level during the period of the study (respectively 91.73, 34.32 ng/mL). However, in the treatment group, the serum PCIII, HA levels were far lower than that of the model rat (P<0.01).
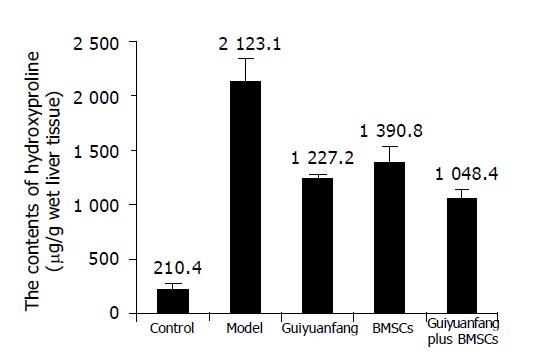
Determination of liver tissue hydroxyproline content confirmed the histological studies, showing a net reduction in total collagen levels. Total liver hydroxyproline levels at model group were 2123.1±218.9 μg/g wet weight, which represent a 10-fold increase, compared to normal livers (P<0.01), and decreased to about 1000 μg/g wet weight in Guiyuanfang or BMSCs group (Figure 1).
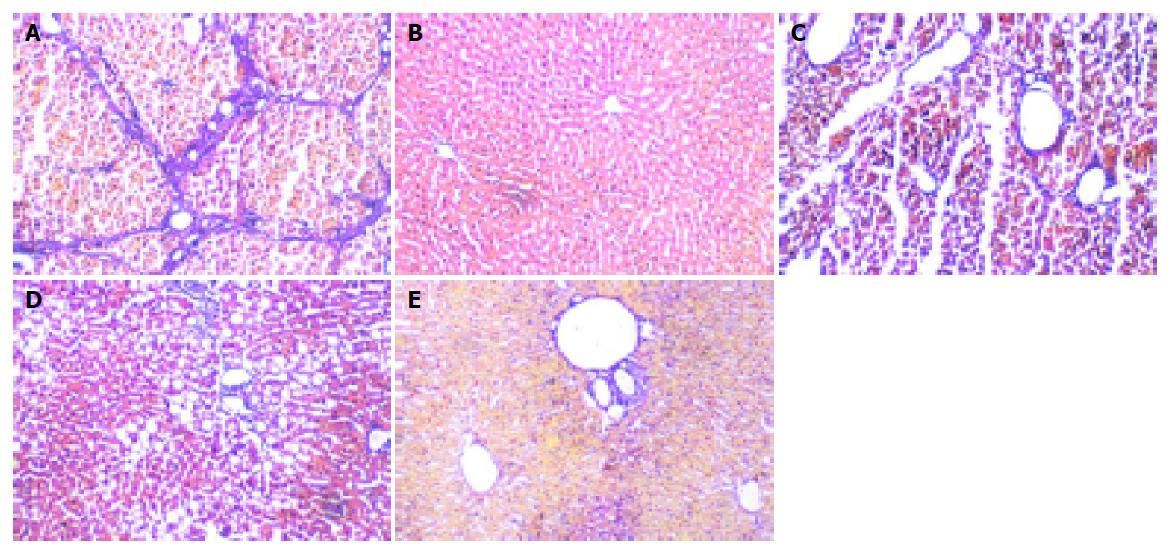
In normal livers, only minimal collagen staining was present; no fibrosis was detected in the group. In model rats, there was obvious nodular fibrosis with deposition of well-delineated fibrosis septa, extensive collagen deposition, which were continuous and extended throughout each section, with mature collagen fibrils bridging portal regions and vascular structures, including perivenular ballooning degeneration of hepatocytes. In Guiyuanfang group, mild fatty change and vacuolation of hepatocytes were observed. The fibrous septa were less well defined and discontinuous and fewer nodules of regenerative parenchyma were noted. In autologous BMSC group, there was evidence of early fibrosis with a number of cells with fibroblast-like morphology in some sections. The appearance of bridging collagen fibers was prevented almost completely in rats treated with Guiyuanfang plus autologous BMSCs. Only occasional short fibril fragments could be visualized. Most liver tissues were restored to normal organization (Figure 2). The χ2 test was used to assess the fibrosis score, and there was significant difference among all treated groups and model group (Table 3).
| Group | n | Grade of liver fibrosis | ||||
| 0 | I | II | III | IV | ||
| Control | 10 | 8 | 2 | 0 | 0 | 0 |
| Model | 10 | 0 | 0 | 0 | 2 | 8 |
| Guiyuanfang | 10 | 0 | 2 | 5 | 2 | 1 |
| MSCs | 8 | 0 | 2 | 3 | 3 | 0 |
| Guiyuanfang plus BMSCs | 8 | 0 | 3 | 4 | 1 | 0 |
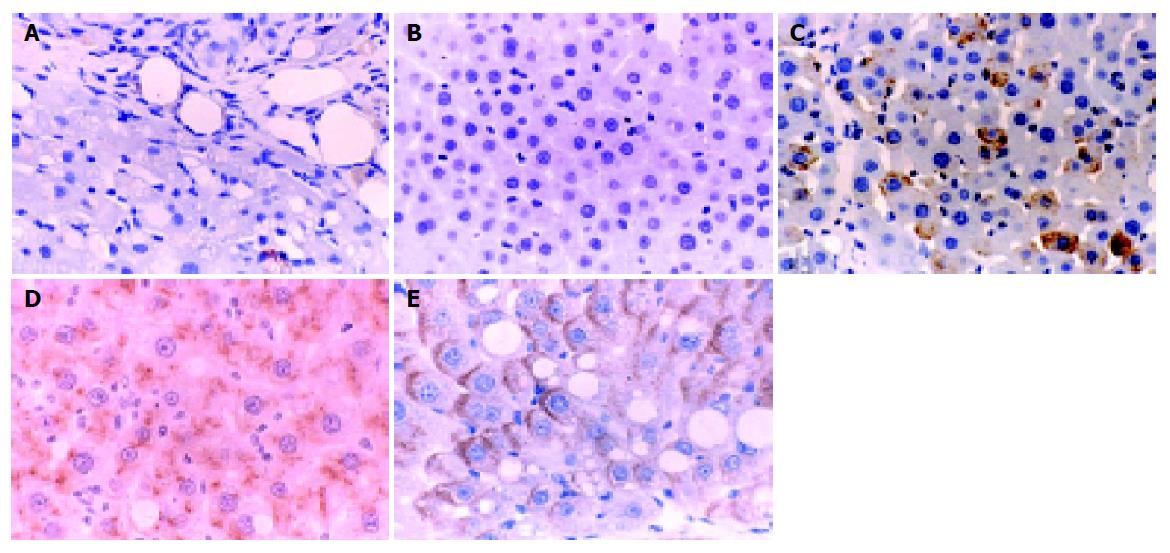
The normal liver tissues showed very weak uPA expression, and Guiyuanfang caused a marked increase of uPA expression in the livers in a dose-dependent manner. The uPA expression in the liver tissues of autologous BMSC transplantation group and Guiyuanfang plus autologous BMSCs transplantation group was the strongest (Figure 3).
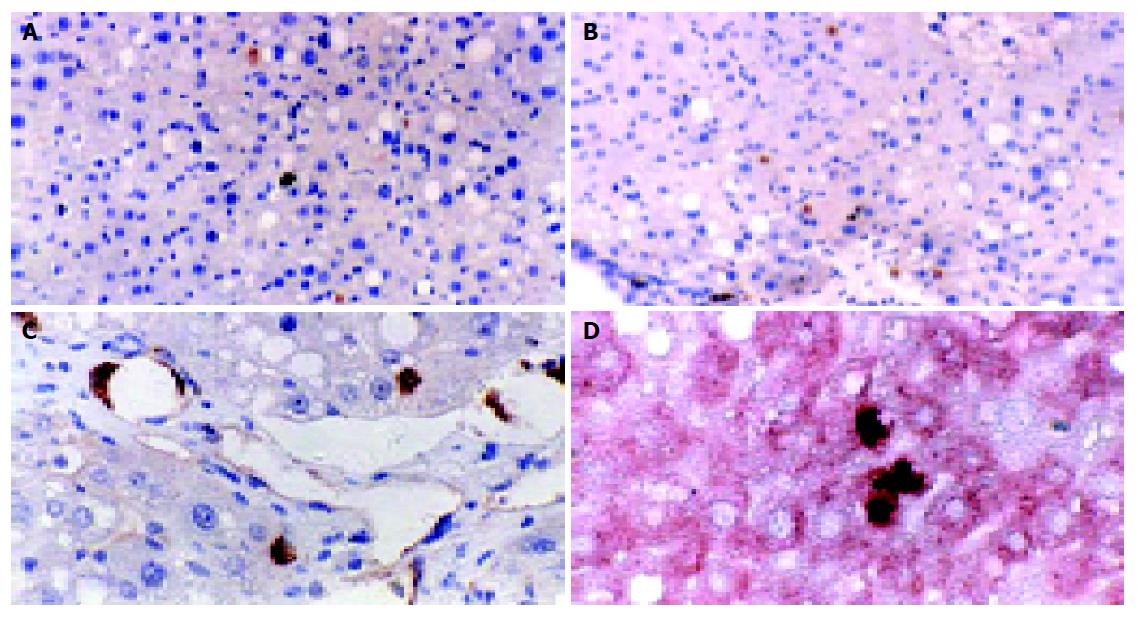
The transplanted cells were predominantly in clusters of 1-3 cells each. Double immunohistochemical staining showed that the BrdU-labeled cells were fully integrated in the liver parenchyma, showing the appearance of hepatocytes, along with the expression of cytokeratin-18, the liver epithelial specific marker. Some BMSCs have differentiated into hepatocytes or endothelial cells (Figure 4).
Chronic diseases and age-associated illness are major challenges in today’s medicine. The pathogenesis of these diseases and symptoms may not only be multifactorial but also could vary from one individual to another. The complex, difficult and changing nature of these chronic diseases has certainly slowed western science’s approach to finding treatments leaving today a large medical need that has not yet been met. The potential usefulness of Chinese medicine embodies the belief that maintaining healthy homeostasis of the body involves the balance of a mixture of chemicals at different organs or tissues. The liver fibrosis is one of these diseases. To find new therapies for liver diseases, the best approach is to integrate traditional and modern approaches. Now cell transplantation has been found to be promising for liver diseases. So integrating Chinese medicine with cell transplantation will provide us with new leads to understand the regulation of homeostasis and further advancement of human medicine.
To the best of our knowledge, this is the first experimental study, investigating TCM and BMSC transplantation on liver fibrosis. To ensure the therapeutic effect, we selected the empiristic formula, Guiyuanfang, which has been used for hundreds of years. Our work showed that Guiyuanfang could improve liver function, protect hepatocytes from toxicant, promote protein synthesis, maintain cytoplasm membrane stable, and provide a good environment for hepatocyte function. It also inhibits inflammatory response, reduces necrotic region, and promotes the phagocytosis of debris and fibrils. Guiyuanfang reverses the liver fibrosis by neutralizing profibrogenic cytokines inhibiting the generation and accumulation of collagen, promoting the degradation of ECM, and improving microcirculation. It is possible that the protective effects of Guiyuanfang may be due to either enhanced collagen breakdown or diminished collagen synthesis. In the study, we observed the obvious increase of uPA expression in the fibrotic liver tissue, after administration of Guiyuanfang. The recovery from fibrosis was correlated with the uPA expression.
Previous work has shown that hepatocyte transplantation can correct liver-based metabolic disorders, prolong the survival of rodents with experimentally-induced acute liver failure, and provide significant hepatic support to animals with hypertension, liver fibrosis, and irreversible cirrhosis[14]. Recently accumulating evidence indicates that bone marrow can differentiate into specific cell types. Transplantation of bone marrow cells in vivo resulted in the appearance of hepatocyte of donor origin in recipient liver[15-18]. Transplanted bone marrow cells can repopulate hepatic and biliary duct epithelia under certain circumstances. Generally the patients with liver fibrosis are likely to have difficulty in producing healthy hepatocytes. There were a lot of solutions reported to enhance bone marrow cell differentiation and proliferation, however, all had their own shortage[19-21], so how to improve conditions to induce hepatocytes from bone marrow cells in vivo and in vitro is a great challenge for the medical scientists. In this study, Guiyuanfang could improve the liver microcirculation, reduce the toxic metabolic, and finally provide a good environment for the transplanted BMSCs to engraft and to proliferate in the fibrotic liver. Although we could not determine whether the initial survival of transplanted cells in the fibrotic liver was different from that in the normal liver, and we were still only able to propagate a small number of hepatocyte-like cells from BMSCs, the present study is a step further in such a direction, and this work still has important implications.
In conclusion, we found that transplanted BMSCs engrafted, survived, and proliferated in the fibrosis livers indefinitely. BMSCs in hepatic fibrogenic environment can differentiate into hepatocytes, endothelial cells. Guiyuanfang can synergize with the BMSCs to improve the recovery from liver fibrosis. Such a possibility will be of great interest in developing treatments for patients with liver fibrosis.
| 1. | Schuppan D, Jia JD, Brinkhaus B, Hahn EG. Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999;30:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Slehria S, Rajvanshi P, Ito Y, Sokhi RP, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology. 2002;35:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, Fox IJ. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gagandeep S, Rajvanshi P, Sokhi RP, Slehria S, Palestro CJ, Bhargava KK, Gupta S. Transplanted hepatocytes engraft, survive, and proliferate in the liver of rats with carbon tetrachloride-induced cirrhosis. J Pathol. 2000;191:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 194] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 720] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 8. | Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 365] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Ott M, Schmidt HH, Cichon G, Manns MP. Emerging therapies in hepatology: liver-directed gene transfer and hepatocyte transplantation. Cells Tissues Organs. 2000;167:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Dorshkind K. Multilineage development from adult bone marrow cells. Nat Immunol. 2002;3:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3924] [Article Influence: 163.5] [Reference Citation Analysis (5)] |
| 12. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1897] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 13. | Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000;31:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (2)] |
| 14. | Fox IJ. Transplantation into and inside the liver. Hepatology. 2002;36:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 750] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 16. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 854] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1638] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 18. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 664] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 19. | Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Mallet VO, Mitchell C, Mezey E, Fabre M, Guidotti JE, Renia L, Coulombel L, Kahn A, Gilgenkrantz H. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002;35:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |