Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.917
Revised: May 9, 2004
Accepted: June 17, 2004
Published online: February 14, 2005
AIM: To establish a new pig model for auxiliary partial orthotopic liver transplantation (APOLT).
METHODS: The liver of the donor was removed from its body. The left lobe of the liver was resected in vivo and the right lobe was used as a graft. After the left lateral lobe of the recipient was resected, end-to-side anastomoses of suprahepatic inferior vena cava and portal vein were performed between the donor and recipient livers, respectively. End-to-end anastomoses were made between hepatic artery of graft and splenic artery of the host. Outside drainage was placed in donor common bile duct.
RESULTS: Models of APOLT were established in 5 pigs with a success rate of 80%. Color ultrasound examination showed an increase of blood flow of graft on 5th d compared to the first day after operation. When animals were killed on the 5th d after operation, thrombosis of hepatic vein (HV) and portal vein (PV) were not found. Histopathological examination of liver samples revealed evidence of damage with mild steatosis and sporadic necrotic hepatocytes and focal hepatic lobules structure disorganized in graft. Infiltration of inflammatory cells was mild in portal or central vein area. Hematologic laboratory values and blood chemical findings revealed that compared with group A (before transplantation), mean arterial pressure (MAP), central venous pressure (CVP), buffer base (BB), standard bicarbonate (SB) and K+ in group B (after portal vein was clamped) decreased (P<0.01). After reperfusion of the graft, MAP, CVP and K+ restored gradually.
CONCLUSION: Significant decrease of congestion in portal vein and shortened blocking time were obtained because of the application of in vitro veno-venous bypass during complete vascular clamping. This new procedure, with such advantages as simple vessel processing, quality anastomosis, less postoperative hemorrhage and higher success rate, effectively prevents ischemia reperfusion injury of the host liver and deserves to be spread.
- Citation: Peng CH, Shi LB, Zhang HW, Peng SY, Zhou GW, Li HW. Establishment of a new pig model for auxiliary partial orthotopic liver transplantation. World J Gastroenterol 2005; 11(6): 917-921
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.917
Orthotopic liver transplantation has become an accepted form of treatment for chronic liver failure, acute liver failure, primary hepatic malignancy not treatable by conventional resection and inborn errors of metabolism due to a liver-based enzyme defect but without parenchymal liver disease[1]. In the middle of the 1990s, with the development of reduced-size liver transplantation, split liver transplantation and partial-living liver transplantation, much attention was paid to auxiliary partial orthotopic liver transplantation (APOLT)[2,3]. APOLT, in which a reduced-size graft orthotopically replaces the resected lobe of the recipient, has been introduced with acceptable results for patients with fulminant hepatic failure (FHF)[4,5]. It has great advantages over standard orthotopic liver transplantation. In APOLT, both the reduced native liver and the reduced graft can obtain optimal accommodation, and a position close to the right atrium may improve hepatic venous outflow of the graft[6]. On the other hand, if the graft is destroyed by some events such as severe rejection or if primary nonfunction occurs, the remnant native liver can sustain the patient’s life and emergency re-transplantation is not necessary[6,7]. Furthermore, when the diseased native liver regenerates with its function fully restored, the withdrawal of immunosuppressive therapy is then possible[5,8], which will decrease the incidence of drug-related side effects and neoplasia, and monitoring requirements[9]. Also, in the preserved native liver future gene therapy is still possible[10].
However, there are few studies of APOLT using large animal models. In this experimental study, we attempted to establish a new pig model of APOLT and evaluated the changes of intraoperative systemic hemodynamics and graft function in the model.
Ten healthy male or female domestic pigs, weighing 22-27 kg, were randomly selected to be either donor group (n = 5) or recipient group (n = 5). Another 5 animals, weighing 75-150 kg, were used as blood supply group. Prior to surgical procedure, the pigs were fasted for 12 h, but were fed water ad libitum.
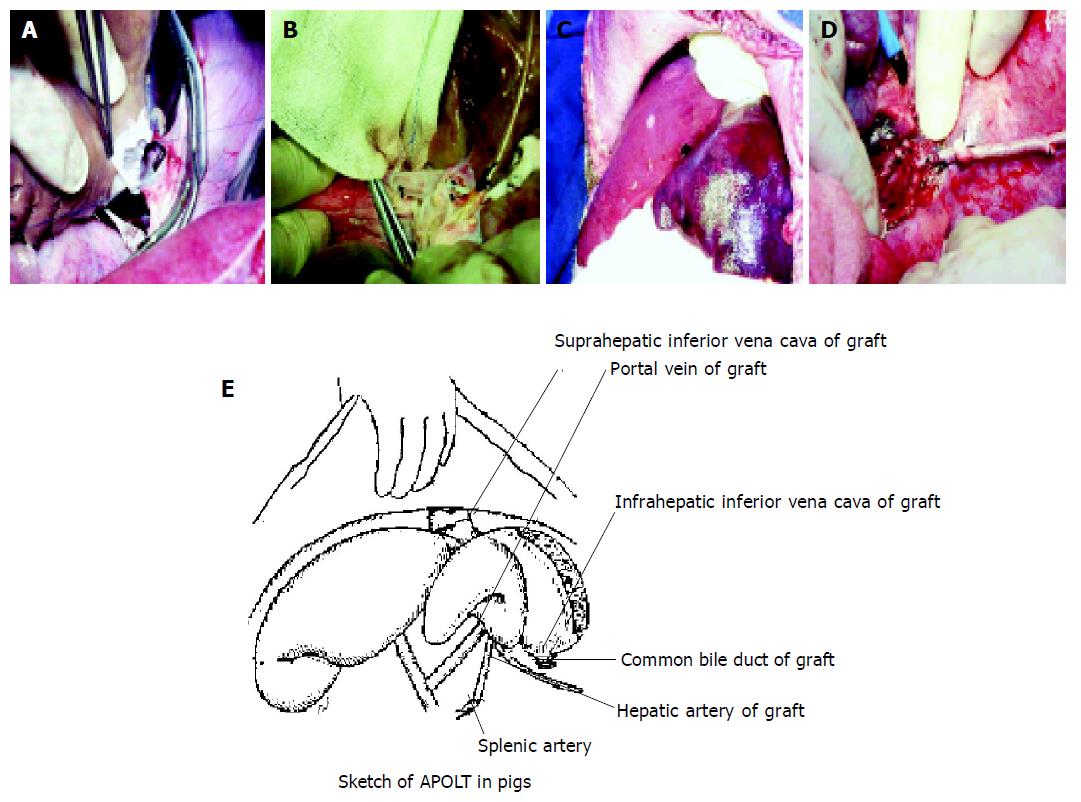
Anesthesia was induced by ketamine intramuscularly with a dose of 20 mg in the donor or recipient. After intubation, inhalation anesthesia was maintained by pentothal sodium. For donor operations, we used a midline laparotomy. The infrahepatic inferior vena cava (IVC), common bile duct, portal vein (PV) and hepatic artery (HA) were dissected and exposed. The hepatic artery was kept in continuity with the celiac trunk and abdominal aorta up to the iliac bifurcation. The whole liver was perfused in situ with 4 °C cold kidney preservation solution via abdominal aorta (1500 mL) and portal vein (2000 mL). Once perfusion was finished, the harvested graft was removed and immersed in a basin filled with cold kidney preservation solution at 4 °C. During bench surgery the gallbladder was resected and the liver splitting was performed with the technique of curettage and aspiration by use of PMOD (Peng’s Multifunctional Operative Dissector). The liver was divided along the right side of the middle hepatic vein and the right branches of the PV. The right liver was used as a partial graft and stored at 4 °C in the same solution bath. During the donor preparation, another surgical team performed the recipient operation. The recipient animal was intubated, anesthetized, and an internal jugular vein catheter was placed for blood sampling, intravenous infusion and central venous pressure (CVP) monitoring. A second catheter was placed in the carotid artery to monitor blood pressure during the procedure. When heparin was intravenously infused with a bolus dose of 2 mg/kg, left hepatectomy was performed and the spleen was resected and removed. Before all hepatic vessels of the recipient were interrupted, an extracorporeal veno-venous bypass (splenic vein and femoral vein to extra jugular vein) was established and the graft (right liver) was placed into the position of left lobe of recipient. The following anastomoses were then made: donor suprahepatic inferior vena cava end-to-side to host suprahepatic inferior vena cava, donor portal vein end-to-side to host portal vein, donor hepatic artery end-to-end to host spleen artery. The donor’s common bile duct was intubated for outside drainage and bile was collected with an extracorporeal bag. The time consumed in suprahepatic inferior vena cava and portal vein anastomoses between donor and recipient was 15-20 min and 10-15 min, respectively. After operation, the donor’s infrahepatic inferior vena cava was ligated. Heat and cold ischemia time were 0 min and 60±4.0 min, respectively. Figure 1A-E shows our APOLT method using the right liver graft. Immunosuppressant was not administered. Antibiotics were administered for three days after operation. During transplantation, arterial blood pressure and central venous pressure were recorded. Buffer base (BB), standard bicarbonate (SB) and pH of arterial blood sample were measured in order to assess the impact of operation on the animals. Color ultrasound examinations were performed 5 d after operation on selected recipients and autopsies were made on all of the recipients. Wedge biopsy specimens of every pig were obtained and stained with hematoxylin-eosin and then examined.
Data were given as mean±SE. Analysis of variance and q test were used to compare the change of hemodynamics and acid-base balance of the recipients in three groups. P<0.05 was considered statistically significant.
Five recipient pigs were operated up on. Four of five (80%) survived for five days, one recipient could not recover from anesthesia, probably due to hyperkalemia and severe acidosis. The hemodynamics of the other four recipients recovered rapidly and stabilized after graft reperfusion. Color ultrasound examination showed an increase of blood flow of graft on 5th d compared to the first day after operation. When animals were killed on the 5th d after operation, thrombosis of hepatic vein (HV) and portal vein (PV) were not found. Histopathological examination of liver samples made at this time revealed evidence of damage with mild steatosis and sporadic necrotic hepatocytes and focal hepatic lobules structure disorganized in graft, especially in a pericentral venous area. Infiltration of inflammatory cells was mild in portal or central vein area.
Hematologic laboratory values and blood chemical findings revealed that mean arterial pressure (MAP), central venous pressure (CVP), buffer base (BB), standard bicarbonate (SB) and K+ decreased when suprahepatic inferior vena cava, infrahepatic inferior vena cava and hepatic hilus were clamped. After reperfusion of the graft, MAP, CVP and K+ restored gradually but BB, SB decreased further (Table 1).
Recent advances in clinical liver transplantation have revealed that auxiliary partial orthotopic liver transplantation (APOLT) is a feasible approach to treat patients with fulminant hepatic failure. Compared to standard orthotopic liver transplantation, APOLT shows us such advantages as the supporting role of remnant native liver against destroyed graft, withdrawal of immunosuppressant and application of small-for-size transplantation, in which remnant native liver can support insufficient graft function until the graft has regenerated sufficiently[5-8,11]. However, there are many unresolved problems in the clinical application of APOLT. The focus problem concerned by many authors was the functional competition resulted from the portal blood flow struggle between the graft and the native liver and the necessity of preemptive transaction of the portal branch to the native liver at the time of transplantation[12-14]. Other problems include deleterious effects of the necrotic liver on the graft function[15] and the difficulty in predicting regeneration of the remnant native liver before operation[16,17]. Thus, it is very important to establish stable experimental models using large animals to clarify these problems.
Welch first performed auxiliary total liver transplantation in 1955 and the first human auxiliary heterotopic liver transplantation was performed by Absolon in 1964. Since then auxiliary liver transplantation became an important branch of liver transplantation especially in treatment of fulminant hepatic failure. Recently, van Hoek reported that the best survival result, after liver transplantation for patients with acute liver failure, could be achieved after APOLT rather than HALT and orthotopic liver transplantation[18]. They also reported that 62% of all patients after auxiliary liver transplantation were free of immunosuppression within 1 year after transplantation. APOLT has been increasingly spread and developed well in Western countries[18-20]. Therefore, it is important to establish a perfect experimental model using large animals for improving the surgical skills of the modern APOLT team. Among the large animal models, pigs are considered the most realistic choice due to their low cost and availability[21]. The purpose of this study was to establish a new and stable pig model of APOLT and observe the changes of intraoperative hemodynamics and graft function in this model.
Taniguchi[22] reported their swine model of APOLT and preliminary data of the model for treatment of ischemic liver injury in 1998, but the operation pattern was very complicated. The characteristic anatomical structure of pigs, quite different from that of humans, lies in its short extrahepatic vena and the intrahepatic bifurcation of the hepatic veins, which lead to difficult anastomoses of hepatic vessels. Our model’s advantages were as follows: (1) Operation was simple and safe. After the graft (right liver) was placed into the position of left lobe of recipient, the following anastomoses were then made: donor suprahepatic inferior vena cava end-to-side to host suprahepatic inferior vena cava, donor portal vein end-to-side to host portal vein, donor hepatic artery end-to-end to host spleen artery. The donor common bile duct was intubated for outside drainage. Furthermore, use of extracorporeal veno-venous bypass before the interruption of all hepatic vessels can markedly decrease the congestion of portal vein and obtain stable hemodynamics. (2) Under the premise of skilled performance of vascular anastomoses, centrifugal pump was employed in extracorporeal veno-venous bypass. The anhepatic phase was controlled within 30 min and ischemia reperfusion injury of the host liver can be effectively prevented (3) Suture was performed on the section of the donor liver to prevent hemorrhage. End-to-side anastomoses of suprahepatic inferior vena cava were performed between the donor liver and recipient liver. Because of the simple vessel processing, unobstructed stoma and little postoperative hemorrhage could easily be achieved.
One of the puzzles of partial liver transplantation is hemorrhage on the section of the donor liver. The quantity of intraoperative bleeding is regarded as a significant risk factor relating to early mortality after liver transplantation. Blood coagulation disorder after reperfusion, incomplete surgical hemostasis and blood leakage at stoma are the main reasons for hemorrhage. In our experiment, hepatic parenchyma was split with forceps fracture method when the donor liver trimming and resection of liver lobes were performed in vitro. Hepatic vein and left branch of portal vein were sutured with Prolene thread under direct view. We recognized that hemorrhage on the section of the donor liver would not happen during and after operation if the graft had vitality. The heparin consumption of pigs was twice as much as that of human when thorough heparinization was obtained. The related check results still stayed at normal levels even if widespread blood permeation had taken place. Therefore, the proper administration of heparin and hemostatic drugs was a key procedure to prevent blood coagulation disorders.
In APOLT, the reperfusion of graft and the metabolic changes during operation consequentially lead to relevant hemodynamic change. Our experiment proved that there were certain rules about hemodynamic change in APOLT: Hemodynamics was stable before the onset of new liver’s blood circulation. MAP decreased and HR speeded up after onset of blood circulation of portal vein of donor liver (compared to preoperation) (P<0.01). The change of CVP was inapparent. We believe that the abrupt decrease of returned blood volume was the main reason for hemodynamic change at this phase. After such treatments as accelerating fluid replacement and blood transfusion, MAP and HR could restore to stability. Plenty of toxin released from recipient liver due to reperfusion injury, hypothermia and plenty of fluid containing potassium in liver or kidney preservation solution, etc., usually resulted in metabolic acidosis and hyperpotassemia after the onset of new liver’s blood circulation. One of the key procedures, that had to be stressed in this model, was the correcting of metabolic acidosis and hyperpotassemia.
The severity of congestion in portal vein system was relevant to two factors in APOLT. One was portal vein’s blockage period. Pigs were considered to be sensitive to the congestion of portal vein system blockage. Obvious intestinal mucosa injury took place 45 min after blockage[22]. Another was retention blood flow in portal vein system. Pigs have short limbs and strong gastrointestinal tract. Blood flow in portal vein is plentiful. Severe congestion in portal vein system would certainly lead to whole body’s hemodynamic disorder. The exsanguine liver period of recipient group all lasted less than 40 min. The changes of MAP, CVP, K+, PCO2, BB and SB took place after the blockage of hepatic hilus, suprahepatic and infrahepatic inferior vena cava (that was anhepatic phase). But the change gradually restored as portal vein was reperfused.
Our experiments proved we have established a new operative and ideal large animal model of APOLT. We predict this model may contribute to experimental or clinic APOLT research.
| 1. | Bramhall SR, Minford E, Gunson B, Buckels JA. Liver transplantation in the UK. World J Gastroenterol. 2001;7:602-611. [PubMed] |
| 2. | Bismuth H, Morino M, Castaing D, Gillon MC, Descorps Declere A, Saliba F, Samuel D. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 183] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Bismuth H, Azoulay D, Dennison A. Recent developments in liver transplantation. Transplant Proc. 1993;25:2191-2194. [PubMed] |
| 4. | Bismuth H, Azoulay D, Samuel D, Reynes M, Grimon G, Majno P, Castaing D. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann Surg. 1996;224:712-724; discussion 724-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Chenard-Neu MP, Boudjema K, Bernuau J, Degott C, Belghiti J, Cherqui D, Costes V, Domergue J, Durand F, Erhard J. Auxiliary liver transplantation: regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure--a multicenter European study. Hepatology. 1996;23:1119-1127. [PubMed] |
| 6. | Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368-375; discussion 375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 426] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Whitington PF, Emond JC, Heffron T, Thistlethwaite JR. Orthotopic auxiliary liver transplantation for Crigler-Najjar syndrome type 1. Lancet. 1993;342:779-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Sudan DL, Langnas AN, Shaw BW. Long-term follow-up of auxiliary liver transplantation for fulminant hepatic failure. Transplant Proc. 1997;29:485-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Ramos HC, Reyes J, Abu-Elmagd K, Zeevi A, Reinsmoen N, Tzakis A, Demetris AJ, Fung JJ, Flynn B, McMichael J. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 171] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Davern TJ, Scharschmidt BF. Gene therapy for liver disease. Dig Dis. 1998;16:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kasahara M, Kiuchi T, Uryuhara K, Takakura K, Egawa H, Asonuma K, Uemoto S, Inomata Y, Tanaka K. Auxiliary partial orthotopic liver transplantation as a rescue for small-for-size grafts harvested from living donors. Transplant Proc. 1998;30:132-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Yabe S, Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, Nishizawa H, Shapiro AM, Yamaoka Y, Tanaka K. Auxiliary partial orthotopic liver transplantation from living donors: significance of portal blood flow. Transplantation. 1998;66:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Uemoto S, Yabe S, Inomata Y, Nishizawa H, Asonuma K, Egawa H, Kiuchi T, Okajima H, Yamaoka Y, Yamabe H. Coexistence of a graft with the preserved native liver in auxiliary partial orthotopic liver transplantation from a living donor for ornithine transcarbamylase deficiency. Transplantation. 1997;63:1026-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kaibori M, Egawa H, Inomata Y, Uemoto S, Asonuma K, Kiuchi T, Varela-Fascinetto G, Matsukura T, Kasahara M, Uryuhara K. Selective portal blood flow diversion in auxiliary partial orthotopic liver transplantation to induce regeneration of the graft. Transplantation. 1998;66:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Inomata Y, Uemoto S, Asonuma K, Egawa H. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 316] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Tanaka K, Uemoto S, Tokunaga Y, Fujita S, Sano K, Nishizawa T, Sawada H, Shirahase I, Kim HJ, Yamaoka Y. Surgical techniques and innovations in living related liver transplantation. Ann Surg. 1993;217:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 436] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Ikegami T, Nishizaki T, Yanaga K, Shimada M, Kakizoe S, Nomoto K, Hiroshige S, Sugimachi K. Changes in the caudate lobe that is transplanted with extended left lobe liver graft from living donors. Surgery. 2001;129:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary versus orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Gubernatis G, Pichlmayr R, Kemnitz J, Gratz K. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg. 1991;15:660-665; discussion 665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Boudjema K, Cherqui D, Jaeck D, Chenard-Neu MP, Steib A, Freis G, Becmeur F, Brunot B, Simeoni U, Bellocq JP. Auxiliary liver transplantation for fulminant and subfulminant hepatic failure. Transplantation. 1995;59:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Busttil RW, Klintmalm GB. Transplantation of the liver. Philadelphia: W.B. Saunders; 1996; 33. |
| 22. | Taniguchi H, Takada Y, Fukunaga K, Yuzawa K, Otsuka M, Todoroki K, Fukao K. Establishment of a swine model for auxiliary partial orthotopic liver transplantation. Transplant Proc. 1998;30:3232-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Edited by Zhu LH