Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.744
Revised: March 1, 2004
Accepted: April 13, 2004
Published online: February 7, 2005
AIM: To investigate the role of cyclin D1, p16 and retinoblastoma in cancerous process of gallbladder carcinomas and to assess the relation between cyclin D1, p16, Rb and the biological characteristics of gallbladder carcinoma.
METHODS: Forty-one gallbladder carcinoma, 7 gallbladder adenoma and 14 chronic cholecystitis specimens were immunohistochemically and histopathologically investigated for the relation of cyclin D1, p16 and Rb with Nevin staging and pathologic grading.
RESULTS: The expression rates of abnormal cyclin D1 in gallbladder carcinoma (68.3%)and gallbladder adenoma(57.1%) were significantly higher than those in chronic cholecystitis (7.1%) (P<0.05). No significant difference was found both among the pathological grades G1, G2 and G3 and among Nevin stagings S1-S2, S3 and S4-S5 of gallbladder carcinoma. The positive rates of p16 (48.8%) and Rb (58.5%) in gallbladder carcinoma were significantly lower compared to those in adenoma (100.0%) and cholecystitis (100.0%) (P<0.05). The positive rates of p16 and Rb in Nevin stagings S1-S2 (80.0% and 90.0%) and S3 (46.2% and 61.5%) gallbladder carcinomas were significantly higher than those in S4-S5 (33.3% and 38.8%) (P<0.05), and those in pathologic grades G1 (54.5% and 81.8%) and G2 (50.0% and 62.5%) gallbladder carcinoma were significantly higher than those in G3 (28.6% and 35.7%) (P<0.05). The protein expression of p16 and Rb had a negative-correlation in gallbladder carcinoma (r = -0.2993, P<0.05), and this negative-correlation was correlated with Nevin staging (P<0.05). Moreover, the protein expression of p16 and cyclin D1 had a negative-correlation in gallbladder carcinoma (r = -0.9417, P<0.05).
CONCLUSION: Cyclin D1 may play a role in the early stage of gallbladder carcinoma. Mutation of p16 and Rb genes might be correlated with progression of gallbladder carcinoma. Analysis of p16 and Rb can estimate the prognosis of gallbladder carcinoma. Expression of p16 and Rb may be correlated with Nevin staging and pathologic grading in gallbladder carcinoma.
- Citation: Ma HB, Hu HT, Di ZL, Wang ZR, Shi JS, Wang XJ, Li Y. Association of cyclin D1, p16 and retinoblastoma protein expressions with prognosis and metastasis of gallbladder carcinoma. World J Gastroenterol 2005; 11(5): 744-747
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.744
Primary carcinoma of gallbladder is a very lethal malignant tumor because of its early metastasis, strong invasion and poor prognosis. It is very important to estimate the malignant degree and invasion tendency in order to guide clinical diagnosis and treatment of gallbladder carcinoma[1,2]. Cyclin D1 is considered as an oncogene and can promote progression of the cell cycle to S by cyclin D-dependent kinases (CDK4/CDK6)-mediated phosphorylation of the retinoblastoma (Rb) protein[3,4]. The activities of CDK4/CDK6 are constrained by p16[4,5]. The onset and progression of gallbladder carcinoma are accompanied with multiple genetic changes that result in qualitative and quantitative alterations in individual gene expression[3-6]. By immunohistochemical methods, we analyzed cyclin D1, p16 and Rb expression levels in gallbladder carcinomas, adenomas and cholecystitis to evaluate their relationships with the pathogenesis, development and metastasis of gallbladder carcinoma.
Sixty-two randomly chosen cholecystectomy specimens included 41 gallbladder carcinomas, 7 gallbladder adenomas and 14 chronic cholecystitis. The Nevin staging of gallbladder carcinoma, and pathologic grading of all cases were determined based on clinical materials. Ten cases were determined as S1-S2, 13 cases as S3 and 18 cases as S4-S5, by Nevin staging. Eleven cases were determined as G1, 16 cases as G2 and 14 cases as G3 by pathologic grading. The polyclonal p16 antibody was rabbit antiserum against human p16 protein (Dako co, USA). The polyclonal Rb antibody was rabbit antiserum against human Rb protein (Santa Cruz, USA). The monoclonal cyclin D1 antibody was mouse antiserum against human cyclin D1 protein (Santa Cruz, USA).
Tissue specimens of gallbladder carcinoma were formalin-fixed and paraffin-embedded, and cut into 5 μm thick sections for staining. The working concentrations of p16, cyclin D1 and Rb antibodies were 1:40, 1:80 and 1:100, respectively. Paraffin-embedded sections of gallbladder carcinoma tissue were dewaxed and dehydrated with ethanol. The sections were incubated with 3 mL/L H2O2-methyl alcohol for 30 min. The slides were incubated overnight with appropriate dilutions of monoclonal antibodies/polyclonal antibodies (McAbs/PcAbs) in PBS (pH 7.8) at 4 °C. After several washing steps, the reactivity was visualized using streptavidin/horseradish peroxidase-conjugated horse anti-mouse or goat anti-rabbit immunoglobulin (Zymed Co) diluted at 1:500 in PBS. Diaminobenzidine and hydrogen peroxide were used as substrates. Control slides were incubated with pre-immune sera or PBS. The protein expression was then scored arbitrarily according to the following scales: -, <25% positive cells; +, 25-50% positive cells; ++, >50-75% positive cells; and +++, >75% positive cells. All steps were carried out at room temperature. All reagents were equilibrated at room temperature.
Two×two contingency table, chi-square test, and correlation analysis were performed. SPSS 8.0 statistical software was used for calculation. P<0.05 was considered statistically significant.
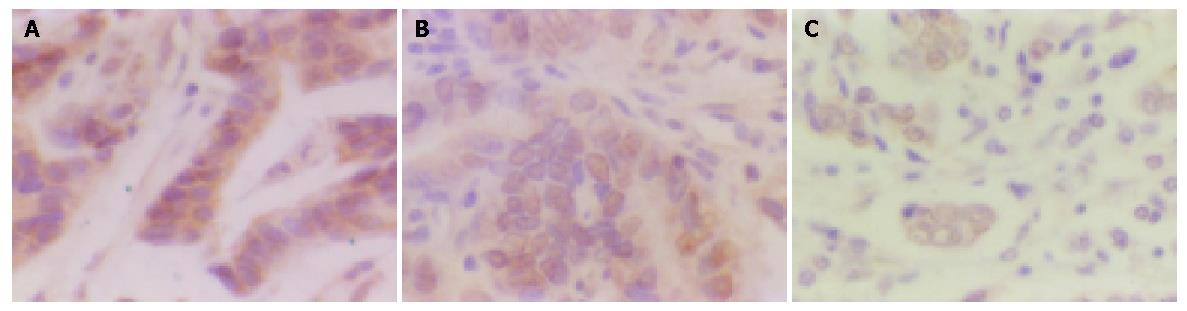
The positive staining for p16 and Rb gene expressed as brown granules, was mainly located in nuclei of tumor cells, partly in cytoplasms (Figure 1: A, B). The positive staining for cyclin D1 gene expressed as brown granules, was distributed mainly in cell nuclei (Figure 1C).
The positive expression rates of cyclin D1, p16 and Rb are shown in Table 1. The abnormal cyclin D1 expression rate in gallbladder carcinoma and gallbladder adenoma was significantly higher than that in chronic cholecystitis (P<0.05). No significant difference was found between gallbladder carcinoma and adenoma, and in pathologic grades G1, G2 and G3, and in Nevin stagings S1-S2, S3 and S4-S5 of gallbladder carcinoma.
| Disease | n | Rb | Cyclin D1 | p16 | |||
| + | % | + | % | + | % | ||
| Gallbladder carcinoma | 41 | 24 | 58.7 | 28 | 68.3 | 20 | 48.8 |
| Adenoma | 7 | 7 | 100 | 4 | 57.1 | 7 | 100 |
| Cholecystitis | 14 | 14 | 100 | 1 | 7.1 | 13 | 92.8 |
The positive expression rates of p16 and Rb in gallbladder carcinomas were significantly higher than those in adenomas and cholecystitis (P<0.05). The positive expression rates of p16 and Rb in Nevin stagings S1-S2 and S3 of gallbladder carcinoma were significantly higher than those in S4-S5 (P<0.05). There was also a significant difference (P<0.05) in p16 and Rb expression between pathologic grades G1 and G3 of gallbladder carcinoma (P<0.05) (Table 2).
| Stages | n | Rb | Cyclin D1 | p16 | ||||
| + | % | + | % | + | % | |||
| Pathologic grading | G1 | 11 | 9 | 81.8 | 8 | 72.7 | 6 | 54.5 |
| G2 | 16 | 10 | 62.5 | 11 | 68.8 | 8 | 50 | |
| G3 | 14 | 5 | 35.7 | 11 | 78.6 | 4 | 28.6 | |
| Nevin staging | S1-S2 | 10 | 9 | 90 | 6 | 60 | 8 | 80 |
| S3 | 13 | 8 | 61.5 | 8 | 61.5 | 6 | 46.2 | |
| S4-S5 | 18 | 7 | 38.8 | 14 | 77.8 | 6 | 33.3 | |
Among the 20 cases of p16-positive gallbladder cancer, 8 were Rb positive. Of the 24 cases of Rb-positive gallbladder cancer, 8 were p16 positive. Tumor suppressor gene p16 was correlated with Rb (χ2 = 5.53, P<0.05; r = -0.2993, P<0.05). There was a negative correlation between p16 and cyclin D1 (χ2 = 6.03, P<0.05; r = -0.9417, P<0.05), but no correlation between Rb and cyclin D1 (χ2 = 1.20) (Table 3).
| p16 | Rb | Cyclin D1 | Total | ||
| + | - | + | - | ||
| + | 8 | 12 | 10 | 10 | 20 |
| - | 16 | 5 | 18 | 3 | 21 |
| Total | 24 | 17 | 28 | 13 | 41 |
It has been accepted that there is a restriction point in cell-cycle progression, the restriction point of G1-S is important. Rb, p16 and cyclin D1 are major restriction factors in the restriction point. The cyclin D1/p16/Rb pathway plays a critical role in tumorigenesis and each component of this pathway may be affected by various malignancies[7-11]. Cell-cycle progression is normally regulated by cyclins and cyclin inhibiting proteins. Progression of cells from G1 to S phase is regulated via pRb phosphorylation by cyclin D complexed with cyclin-dependent kinases (CDK) 4 and 6, which are in turn regulated by CDK inhibitors, such as p16INK4 protein[12]. pRb is underphosphorylated throughout G1 phase and phosphorylated just before S phase[12,13]. Hypophosphorylated pRb arrests cells in G1 phase, and phosphorylation relieves this inhibition resulting in S phase entry[12-14]. p16INK4 is associated with the cyclin D-CDK4 complex, preventing pRb phosphorylation and consequently, S phase entry[15-18]. Dysregulation of the p16INK4/pRb/cyclin D1 pathway has been reported in numerous tumor types[19,20]. Inactivation of tumor suppressor gene products pRb and p16INK4 protein is a common event in human cancers[9].
P16 gene located on chromosome 9p21, is a new tumor suppressor gene, which was identified by an American molecular geneticist in 1995 and is also called multiple tumor suppressor 1 (MTS1) for its suppressing function on multiple tumors[16]. Rb gene is the first tumor suppressor gene located at chromosome 13q14 identified by the location cloning method. The product of Rb is a nuclear phosphoprotein. Loss of pRb has been demonstrated in a variety of cancers, including gastric, pancreatic and bladder cancers, small cell lung and colorectal carcinomas[21-25].
Our study showed that the positive expression rates of p16 and Rb in gallbladder carcinoma were significantly lower than those in adenoma and cholecystitis. The positive expression rates of p16 and Rb in Nevin stagings S1-S2 and S3 of gallbladder carcinoma were significantly higher than those in S4-S5. There was also a significant difference in p16 and Rb expressions between pathologic grades G1 and G3 of gallbladder carcinoma. These results suggest that loss and mutation of p16 play an important role in gallbladder carcinoma progression, and there is a consanguineous relation between p16 and invasion and metastasis of gallbladder carcinoma. p16 gene can inhibit the development of gallbladder carcinoma.
The overexpression of cyclin D1 has been reported in a wide range of human cancers[26-28]. In our study the abnormal cyclin D1 expression rates in gallbladder carcinoma and adenoma were significantly higher than those in chronic cholecystitis. No significant difference was found in cyclin D1 expression between gallbladder carcinoma and adenoma. Similarly, there was no marked difference among the pathologic grades G1, G2 and G3 and among Nevin stagings S1-S2, S3 and S4-S5 of gallbladder carcinoma. Hui et al[29] have suggested that cyclin D1 overexpression is an early event in gallbladder carcinogenesis and could independently predict the prognosis for patients with resectable gallbladder carcinoma.
Among the 20 cases of p16-positive gallbladder cancer, 8 were Rb positive. Of 24 cases of Rb-positive gallbladder cancer, 8 were p16 positive. Tumor suppressor gene p16 is correlated with Rb. Therefore, inactivation of pRb may stimulate cells to increase p16INK4 expression, which inhibits the activity of CDK4 and inactivates cyclin D1-CDK4 complex. On the contrary, pRb overexpression stimulates cells to increase p16INK4 protein loss, which enhances the activity of CDK4, thus inactivating excrescent pRb.
There is a negative correlation between p16 and cyclin D1. A close association of cyclin D1 overexpression with p16INK4 protein loss has been found in bladder cancer[30] and hepatocellular carcinoma[31,32]. Negative expression of p16INK4 protein and positive expression of cyclin D1 protein are significantly correlated to the high invasion and metastasis of tumor and the poor survival of patients.
In conclusion, disruption of the cyclin D1/p16INK4-pRb pathway plays an important role in the progression of gallbladder carcinoma. Loss or decreased expression of p16 and pRb has an obvious correlation with malignant degree and metastasis of gallbladder carcinoma. Cyclin D1 overexpression is an early event in gallbladder carcinogenesis.
Edited by Kumar M and Wang XL
| 1. | Fan YZ, Zhang JT, Yang HC, Yang YQ. Expression of MMP-2,TIMP-2 protein and the ratio of MMP-2/TIMP-2 in gallbladder carcinoma and their significance. World J Gastroenterol. 2002;8:1138-1143. [PubMed] |
| 2. | Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg. 2004;8:83-89; discussion 89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Lerma E, Esteller M, Herman JG, Prat J. Alterations of the p16/Rb/cyclin-D1 pathway in vulvar carcinoma, vulvar intraepithelial neoplasia, and lichen sclerosus. Hum Pathol. 2002;33:1120-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sdek P, Zhang Z, Cao J. Influence of HPV16 on expression of Rb, p16 and cyclin D1 in oral epithelial cell. Zhonghua KouQiang YiXue ZaZhi. 2002;37:84-86. [PubMed] |
| 5. | Krämer A, Schultheis B, Bergmann J, Willer A, Hegenbart U, Ho AD, Goldschmidt H, Hehlmann R. Alterations of the cyclin D1/pRb/p16(INK4A) pathway in multiple myeloma. Leukemia. 2002;16:1844-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gerdes B, Ramaswamy A, Ziegler A, Lang SA, Kersting M, Baumann R, Wild A, Moll R, Rothmund M, Bartsch DK. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Hwang CF, Cho CL, Huang CC, Wang JS, Shih YL, Su CY, Chang HW. Loss of cyclin D1 and p16 expression correlates with local recurrence in nasopharyngeal carcinoma following radiotherapy. Ann Oncol. 2002;13:1246-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Cho NH, Kim YT, Kim JW. Alteration of cell cycle in cervical tumor associated with human papillomavirus: cyclin-dependent kinase inhibitors. Yonsei Med J. 2002;43:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta. 2002;1602:73-87. [PubMed] |
| 10. | Güner D, Sturm I, Hemmati P, Hermann S, Hauptmann S, Wurm R, Budach V, Dörken B, Lorenz M, Daniel PT. Multigene analysis of Rb pathway and apoptosis control in esophageal squamous cell carcinoma identifies patients with good prognosis. Int J Cancer. 2003;103:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Choi YL, Park SH, Jang JJ, Park CK. Expression of the G1-S modulators in hepatitis B virus-related hepatocellular carcinoma and dysplastic nodule: association of cyclin D1 and p53 proteins with the progression of hepatocellular carcinoma. J Korean Med Sci. 2001;16:424-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Beasley MB, Lantuejoul S, Abbondanzo S, Chu WS, Hasleton PS, Travis WD, Brambilla E. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Yoo J, Park SY, Kang SJ, Shim SI, Kim BK. Altered expression of G1 regulatory proteins in human soft tissue sarcomas. Arch Pathol Lab Med. 2002;126:567-573. [PubMed] |
| 14. | Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol. 2002;33:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1955] [Cited by in RCA: 1894] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 16. | Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2515] [Cited by in RCA: 2580] [Article Influence: 78.2] [Reference Citation Analysis (4)] |
| 17. | Bartkova J, Thullberg M, Slezak P, Jaramillo E, Rubio C, Thomassen LH, Bartek J. Aberrant expression of G1-phase cell cycle regulators in flat and exophytic adenomas of the human colon. Gastroenterology. 2001;120:1680-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Azechi H, Nishida N, Fukuda Y, Nishimura T, Minata M, Katsuma H, Kuno M, Ito T, Komeda T, Kita R. Disruption of the p16/cyclin D1/retinoblastoma protein pathway in the majority of human hepatocellular carcinomas. Oncology. 2001;60:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Shi YZ, Hui AM, Li X, Takayama T, Makuuchi M. Overexpression of retinoblastoma protein predicts decreased survival and correlates with loss of p16INK4 protein in gallbladder carcinomas. Clin Cancer Res. 2000;6:4096-4100. [PubMed] |
| 20. | Feakins RM, Nickols CD, Bidd H, Walton SJ. Abnormal expression of pRb, p16, and cyclin D1 in gastric adenocarcinoma and its lymph node metastases: relationship with pathological features and survival. Hum Pathol. 2003;34:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Raghavan D. Molecular targeting and pharmacogenomics in the management of advanced bladder cancer. Cancer. 2003;97:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Zhang R, Zhang JJ, He ZG, Cheng SJ, Gao YN. Research advances on bladder cancer associated genes. AiZheng. 2003;22:104-107. [PubMed] |
| 23. | Gregorc V, Ludovini V, Pistola L, Darwish S, Floriani I, Bellezza G, Sidoni A, Cavaliere A, Scheibel M, De Angelis V. Relevance of p53, bcl-2 and Rb expression on resistance to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2003;39:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Peiró G, Diebold J, Löhrs U. CAS (cellular apoptosis susceptibility) gene expression in ovarian carcinoma: Correlation with 20q13.2 copy number and cyclin D1, p53, and Rb protein expression. Am J Clin Pathol. 2002;118:922-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kumar RV, Kadkol SS, Daniel R, Shenoy AM, Shah KV. Human papillomavirus, p53 and cyclin D1 expression in oropharyngeal carcinoma. Int J Oral Maxillofac Surg. 2003;32:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Lim SC, Zhang S, Ishii G, Endoh Y, Kodama K, Miyamoto S, Hayashi R, Ebihara S, Cho JS, Ochiai A. Predictive markers for late cervical metastasis in stage I and II invasive squamous cell carcinoma of the oral tongue. Clin Cancer Res. 2004;10:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Cheung TH, Yu MM, Lo KW, Yim SF, Chung TK, Wong YF. Alteration of cyclin D1 and CDK4 gene in carcinoma of uterine cervix. Cancer Lett. 2001;166:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Hui AM, Li X, Shi YZ, Takayama T, Torzilli G, Makuuchi M. Cyclin D1 overexpression is a critical event in gallbladder carcinogenesis and independently predicts decreased survival for patients with gallbladder carcinoma. Clin Cancer Res. 2000;6:4272-4277. [PubMed] |
| 30. | Yang CC, Chu KC, Chen HY, Chen WC. Expression of p16 and cyclin D1 in bladder cancer and correlation in cancer progression. Urol Int. 2002;69:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Huang GX, Cheng RX, Feng DY. p16 and cyclinD1 protein expression and p16 gene mutation in primary human hepatocellular carcinoma. Hunan YiKe DaXue XueBao. 2001;26:527-530. [PubMed] |
| 32. | Ito Y, Matsuura N, Sakon M, Miyoshi E, Noda K, Takeda T, Umeshita K, Nagano H, Nakamori S, Dono K. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology. 1999;30:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 4.7] [Reference Citation Analysis (1)] |