INTRODUCTION
Growing evidence from both animal experiments and clinical observations indicates that apoptosis, a form of cell death that is distinct from necrosis, plays a key role in intestinal ischemia-reperfusion injury[1,2]. The signal transduction pathway that leads to this post-ischemic intestinal apoptosis, however, remains unclear. Recently, cell biology studies have demonstrated that c-Jun N-terminal kinase (JNKs)/stress-activated protein kinase (SAPK) and p38 mitogen-activated protein kinase (MAPK), two members of the MAPK family, are activated by a variety of cellular stresses[3,4] and that their activations result in apoptosis and cell death[5-11]. It has also been known recently that intestinal I/R, an extreme pathological stress to the gut, activates p38 MAPK[12]. However, whether activation of p38 MAPK plays a role in intestinal I/R apoptosis has not been determined. The objectives of the present studies were to determine the time course of p38 MAPK activation in vivo in I/R rat model, establish a direct link between p38 MAPK activation and intestinal epithelial cell apoptosis after intestinal I/R, and define the effects of inhibition of p38MAPK activation and I/R-induced cell apoptosis by specific pharmacological agents on intestinal epithelial barrier functional damage.
MATERIALS AND METHODS
Animal model and experimental design
Ninety healthy male Wistar rats weighing 220±20 g (Animal Centre, Chinese Academy of Military Medical Science, Beijing) were used in this study. Rats were housed in wire-bottomed cages placed in a room illuminated from 08:00 AM to 20:00 PM (12:12 h light-dark cycle) and maintained at 21±1 °C. Rats were allowed access to water and chow ad libitum. After they were anesthetized by 30 g/L sodium pentobarbital (40 mg/kg), and a laparotomy was performed. The superior mesenteric artery (SMA) was identified and freed by blunt dissection. A micro-bulldog clamp was placed at the root of SMA to induce complete cessation of blood flow for 45 min, and thereafter the clamp was loosened to form reperfusion injury[13]. The animals were randomly divided into sham-operation group (C), I/R vehicle group (R) and SB 203580 pre-treated group (S). According to different periods after reperfusion, groups R and S were further divided into 0.25, 0.5, 1, 2, 6, 12 and 24 h subgroups. In group R and S, 0.15 mL saline or 0.15 mL saline plus SB 203580 (250 μg/rat) was injected right before SMA occlusion via tail vein for pre-treatment. In group C, SMA was separated but without occlusion, and samples were taken after exposure to SMA for 45 min. In groups R and S, rats were sacrificed at different time points after reperfusion, and blood samples and intestinal tissue biopsies were taken. Blood samples were centrifuged and serum was frozen to measure plasma D-lactate. A small piece of tissue samples was fixed with 100 g/L neutral buffered formalin for immunohistochemical detection of intestinal epithelial apoptosis. The rest of tissue samples were placed in liquid nitrogen for Western immunoblotting detection of p38 MAPK activities.
Measurement of plasma D-lactate
The level of plasma D-lactate was measured with modified Brandt’s method. Briefly, heparinized blood was centrifuged at 3200 r/min for 10 min and 2 mL of the plasma was deproteinized with 0.2 mL perchloric acid (PCA) (1/10 vol), mixed and kept in an ice bath for 10 min. The denatured protein solution was centrifuged at 3200 r/min for 10 min and the supernatant was removed. To 1.4 mL of supernatant, 0.12 mL KOH was added and mixed for 20 s. Precipitant KCLO4 was removed by centrifugation at 3200 r/min for 10 min. The supernatant and neutralized-protein-free plasma were used to measure the absorbency at 304 nm. Plasma D-lactate concentration was expressed as mmol/L.
Histological staining
Formalin-fixed, paraffin-embedded intestinal samples were also cut into 5-μm thick sections, deparaffinized in xylene, rehydrated in graded ethanol, and then stained with haematoxylin-eosin (HE) for histological observation under light microscope.
In situ detection of cell death
The apoptotic cells of post-ischemic intestinal tissue were detected by the terminal deoxynucleotidyl transferase (TdT)-mediated dUDP-biotin nick end labeling (TUNEL) method[14]. In situ cell death detection kit (POD) was purchased from Roche Inc., USA. Specimens were dewaxed and immersed in phosphate-buffered saline containing 3 g/L hydrogen peroxide for 10 min at room temperature and then incubated with 20 μg/mL proteinase K for 15 min at room temperature. Seventy-five microliters of equilibration buffer was applied directly onto the specimens for 10 min at room temperature, followed by incubation with 55 μL of TdT enzyme at 37 °C for 1 h. The reaction was terminated by transferring the slides to prewarmed stop/wash buffer for 30 min at 37 °C. The specimens were covered with a few drops of rabbit serum and incubated for 20 min at room temperature and then covered with 55 μL of anti-digoxigenin peroxidase and incubated for 30 min at room temperature. Specimens were then soaked in Tris buffer containing 0.2 g/L diaminobenzidine and 0.2 g/L hydrogen peroxide for 1 min to achieve color development. Finally, the specimens were counterstained by immersion in hematoxylin. The cells with clear nuclear labeling were defined as TUNEL-positive cells. The apoptotic cell rate (apoptotic index) was calculated as percentage of TUNEL-positive cells using the following formula: the number of TUNEL-positive cell nuclei / (number of TUNEL-positive cell nuclei + the number of total cell nuclei) ×100.
p38 MAP kinase activity assay
p38 MAP kinase activity assay was performed using a P38 MAPK assay kit (cell signaling technology) according to the manufacturer’s instructions. In brief, intestinal tissue (100 mg) was homogenized in 1 mL ice-cold cell lysis buffer (20 mmol/L Tris, pH 7.5, 150 mmol/L NaCL, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton x-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerolphosphate, 1 mmol/L Na3VO4, 1 mg /mL Leupeptin, 1 mmol/L PMSF). The lysates were then sonicated on ice and centrifuged at 15000 g for 10 min at 4 °C. Immunoprecipitation was performed by adding 20 μL of resuspended immobilized monoclonal antibody against phospho-p38 MAP kinase (Thr 180/Tyr 182) to 200 μL cell lysate containing 200 μg protein. The mixture was incubated with gentle rocking overnight at 4 °C. After centrifugation at 10000 g at 4 °C for 2 min, the pellets were washed twice with lysis buffer and twice with kinase buffer (25 mmol/L Tris, pH 7.5, 5 mmol/L β-glycerolphosphate, 2 mmol/L DTT, 0.1 mmol/L Na3VO4, 10 mmol/L MgCL2 ). Kinase reactions were carried out in the presence of 200 μmol/L of ATP and 2 μg of ATF-2 fusion protein at 30 °C for 30 min. After incubation, the samples were separated by SDS-PAGE, and ATF-2 phosphorylation was measured by Western immunoblotting using a monoclonal antibody against phosphorylated ATF-2, followed by an enhanced chemiluminescent detection.
Statistical analysis
All values were expressed as mean±SE. The statistical significance was determined by one-way analysis of variance (ANOVA), followed by the Student Newman-Keuls multiple comparison test. P<0.05 was considered as statistically significant.
RESULTS
Ischemia - reperfusion activation of p38 MAPK and its blockade by SB 203580
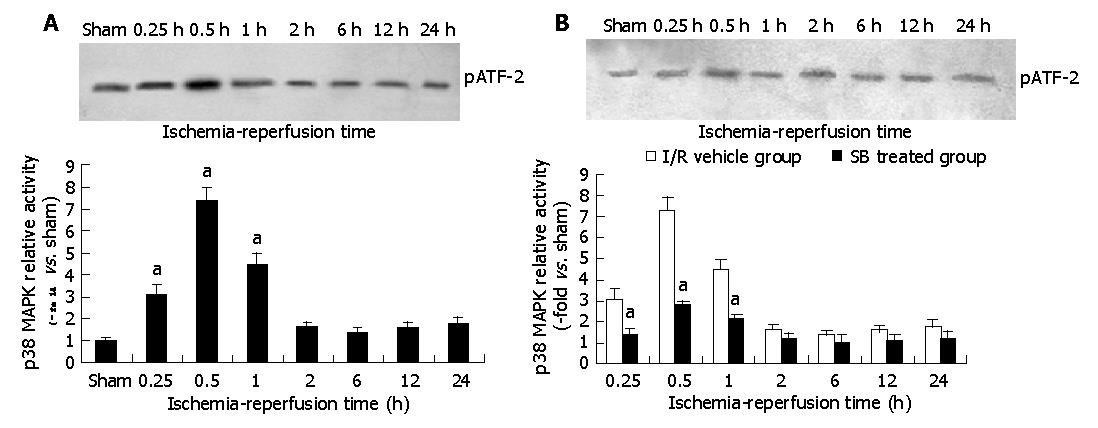
Effect of in vivo intestinal I/R on p38 MAPK activity and its inhibition by SB 203580 were determined using a p38 MAPK assay kit. The data showed that p38 MAPK was markedly activated in intestinal epithelial cells subjected to I/R. A 3.8-fold increase in p38 MAPK activity was observed at 15 min after reperfusion. After 30 min of reperfusion, p38 MAPK activity reached a peak level of 7.3-fold increase and gradually returned to control level thereafter. Treatment with SB 203580 at the dose regime selected virtually abolished p38 MAPK activation and brought p38 MAPK activity to a level that was not statistically different from those seen in intestinal epithelia subjected to sham intestinal I/R (Figures 1A, B). These results demonstrated that p38 MAPK was activated by I/R, and its activation could be inhibited by SB 203580 at the dose regime selected in the present experiment.
Figure 1 Changes of p38 mitogen-activated protein kinase activities in intestinal tissue after I/R (A) and of different groups after I/R (B).
aP<0.05 vs I/R vehicle group.
In situ determination of apoptotic intestinal cells in I/R and its inhibition by SB 203580
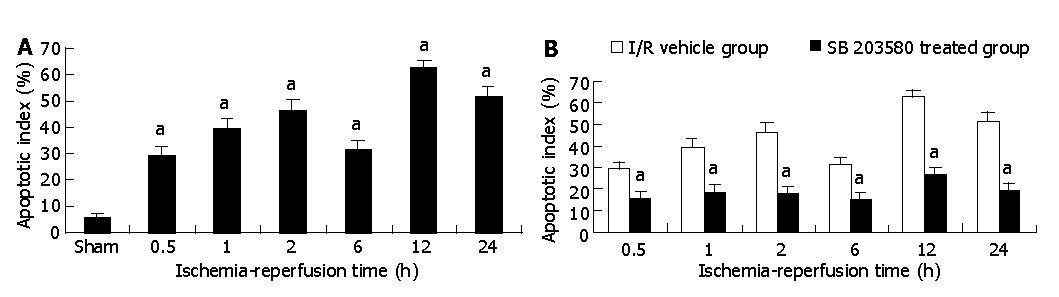
The immunohistochemical staining of small intestinal tissue before and after I/R was evaluated and summarized. Before I/R, few TUNEL-positive cells were observed at the villus surface. However, when ischemic intestinal tissue was reperfused, TUNEL-positive cells of the villus were markedly increased compared with the sham-operation group (P<0.05). The number of TUNEL-positive cells increased 5-fold and 6.7-fold at 30 min and 60 min after I/R (29.83±3.43% vs 5.83±1.47% at 30 min and 39.33±4.32% vs 5.83±1.47% at 60 min, P<0.05), and reached its peak value at 12 h after I/R, which was an increase of 10.72-fold as compared with those in sham-operation group (62.50±3.08% vs 5.83±1.47 %, P<0.01). Treatment with SB 203580 abolished p38 MAPK activity, the number of TUNEL-positive cells significantly reduced to 26.72±2.39 % in SB 203580 pre-treated group compared with 62.50±3.08 % in I/R vehicle group at 12 h after I/R (P<0.05, Figures 2A, B).
Figure 2 Apoptotic cell rate in intestinal tissue after I/R in I/R vehicle group (A) and apoptotic cells rate in intestinal tissue after I/R in I/R vehicle and SB 203580 treated groups (B).
aP<0.05 vs I/R vehicle group.
Effect of p38 MAPK inhibition on intestinal histological damage and plasma D-lactate level after I / R
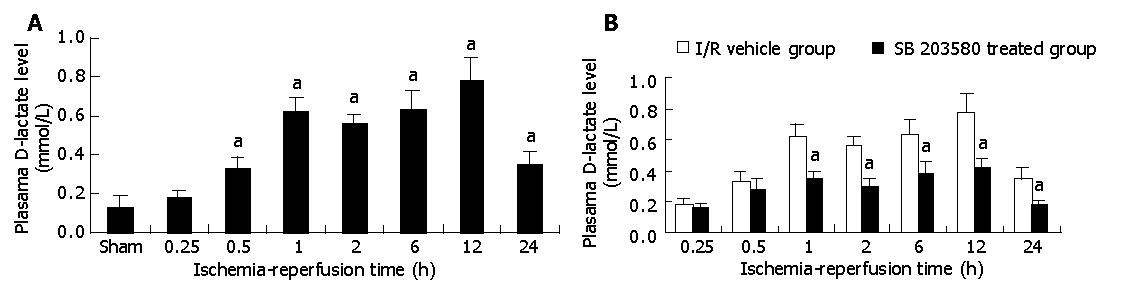
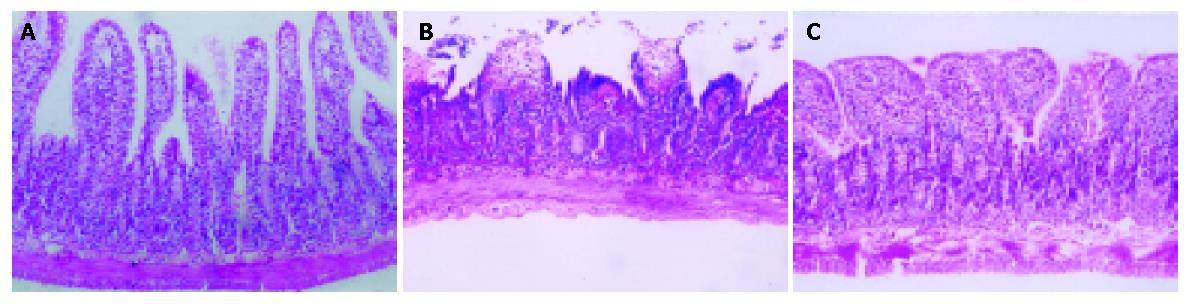
As shown in Figure 3, 45 min after intestinal ischemia followed by reperfusion resulted in significant intestinal histological damage and plasma D-lactate level elevation. Plasma D-lactate level elevated from 15 min after reperfusion in I/R vehicle group, and reached its peak at 12 h, which was an increase of 6-fold as compared with those in sham-operation group (0.78±0.15 mmol /L vs 0.13±0.06 mmol /L, P<0.05) , and then gradually decreased. This increased plasma D-lactate level in I/R vehicle group was significantly decreased by treatment with SB 203580 (0.42±0.17 mmol /L vs 0.78±0.15 mmol /L, P<0.05). HE staining showed the damage of intestinal epithelial cells, hemorrhage and necrosis accompanied by inflammatory cell infiltration into the intestinal wall. However, histological structure of intestinal mucosa was significantly improved after treatment with SB 203580 (Figures 4A-C).
Figure 3 Plasma D-lactate level in intestinal tissue after I/R in I/R vehicle group (A) and plasma D-lactate level in intestinal tissue after I/R in I/R vehicle and SB 203580 treated groups (B).
aP<0.05 vs I/R vehicle group.
Figure 4 Villus structure.
A: Before I/R in sham-operation group; B: Structural changes of the villus after I/R in I/R vehicle group; C: Structural changes of the villus after I/R in SB 203580 treated group.
DISCUSSION
Intestinal ischemia-reperfusion injury may cause significant structural alteration of the intestinal wall including disruption of intestinal epithelial barrier by inducing intestinal epithelial programmed cell death[15,16]. Recently, several mechanisms have been proposed for ischemia-reperfusion injury. The first event is an influx of calcium into cells and a redistribution of intracellular calcium pools with an increase of the free form (biologically active) in the cytosol[17,18]. The increased calcium level manifests its toxic effects on cells by altering mitochondrial respiratory function and energy metabolism, resulting in serious damage to cells. The second event is the generation of oxygen free radicals. Free radicals and calcium work in concert to destroy lipids and thereby destroy membrane structures including both the cell membrane and mitochondrial membrane[19]. The third event is a “no reflow” phenomenon. This condition may contribute to increased neutrophil adherence to damaged endothelium, and expression of P-selectin as well as intercellular adhesion molecule-1(ICAM-1), promoting microcirculation disturbance, which is thought to be a major mechanism of ischemia -reperfusion injury.
Although it is well known that intestinal epithelial cells are highly sensitive to ischemia-reperfusion injury, the relationship between ischemia-reperfusion induced apoptosis and the injury mechanisms involved in the signal transduction remains largely unclear. Some studies demonstrate that MAPK family plays an important role in intracellular signal transduction in response to extracellular stimuli[20-22], such as, ischemia-reperfusion. MAPK family members are activated by dual phosphorylation of tyrosine and threonine in response to extracellular stimuli. Once activated, these kinases are translocated to nuclei, where they phosphorylate and activate different transcription factors and transactivate target genes. In this group of kinases, classical extracellular signal-regulated kinase is mainly stimulated by growth factors, and is associated with proliferation. On the other hand, p38 MAPK and JNK have been reported to be activated by a variety of cellular stresses, such as inflammatory cytokines, lipopolysaccharides, heat shock, osmotic stress, and ischemia- reperfusion[23,24].
The ischemia-reperfusion process differentially activates MAPK. In hepatic ischemia-reperfusion, p38 MAPK and JNK are activated by reperfusion after ischemia. Ma et al.[25] reported that the administration of SB 203580 decreased myocardial apoptosis and improved cardiac function after myocardial ischemia -reperfusion by inhibiting p38 MAPK. In our study, ischemia followed by reperfusion resulted in marked activation of p38 MAPK, with 7.3-fold activation achieved 30 min after ischemia-reperfusion. These results also lead us to hypothesize that the strong activation of p38 MAPK by reperfusion might play a more significant role in subsequent intestinal injury than previously realized.
Another important discovery from the present study is that administration of a p38 MAPK inhibitor, SB 203580, markedly reduced post-ischemic intestinal apoptosis. Although previous studies have demonstrated that p38 MAPK plays a key role in apoptosis in a variety of cell culture systems[26], and that ischemia- reperfusion activates p38 MAPK in animal models[27]. Whether or not p38 MAPK activation in ischemic intestinal tissue is involved in post-ischemic intestinal cell apoptosis has not been directly determined. Our results provide the evidence that p38 MAPK is a key factor in signal transduction leading to intestinal apoptosis after ischemia-reperfusion. However, it should be noted that although administration of SB 203580 blocked p38 MAPK activation, it failed to induce complete inhibition of apoptosis caused by ischemia-reperfusion. These results suggest that other signal transduction pathways, such as JNK/SAPK, may also contribute to post-ischemic intestinal cell apoptosis.
In addition, we also demonstrated that administration of SB 203580 significantly attenuated post-ischemic intestinal histological structural damage and decreased plasma D-lactate level. D-lactate is the stereoisomer of mammalian L (+)-lactate and is produced by bacterial fermentation. Since mammals do not produce D-lactate and possess the enzyme system to rapidly metabolize D-lactate[28,29], the released D-lactate will pass through the gut barrier and liver in an unchanged form and appears in the peripheral blood. As intestinal ischemia injury causes mucosal injury and subsequent bacterial proliferation, D-lactate is released from gut into the circulation. Thus, the changes of serum D-lactate are used as a predictor of intestinal ischemia reperfusion injury. In this study, serum D-lactate level elevation and intestinal mucosal damage were observed after ischemia reperfusion, and inhibition of p38 MAPK by SB 203580, D-lactate was not significantly increased and intestinal mucosal histological damage was improved, indicating that inhibition of p38 MAPK might exert an important protective effect on the mucosal barrier and decrease the permeability.
In summary, intestinal ischemia- reperfusion, a real pathological stress to the gut, results in significant activation of p38 MAPK. This provides the direct evidence that activation of p38 MAPK plays a key role in the signal transduction pathway mediating intestinal apoptosis after ischemia-reperfusion. Inhibiting p38 MAPK, which reduces intestinal apoptosis associated with p38 MAPK activation, significantly improves post-ischemic intestinal epithelial barrier functional recovery.