Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7702
Revised: April 23, 2005
Accepted: April 25, 2005
Published online: December 28, 2005
- Citation: Dohmen K, Wen CY, Nagaoka S, Yano K, Abiru S, Ueki T, Komori A, Daikoku M, Yatsuhashi H, Ishibashi H. Fenofibrate-induced liver injury. World J Gastroenterol 2005; 11(48): 7702-7703
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7702.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7702
Fenofibrate is a member of such fibrate class agents as bezafibrate and it work as a ligand of PPARα, and also shows a potent triglyceride-lowering effect. The elevation of aminotransferase levels has been frequently observed after the administration of fenofibrate and this phenomenon is considered to be non-pathological because fenofibrate activates the gene expression of the aminotransferases. Recently, fenofibrate has been used not only for hypercholesterolemia but also for primary biliary cirrhosis (PBC)[1,2]. However, the occurrence of liver injury induced by fenofibrate has not yet been reported written in the English literature. We herein report a rare case of liver injury due to the oral use of this drug.
A 66-year-old Japanese female patient was admitted to undergo a further examination with a nearly 20-year history of liver dysfunction. She had been previously treated with 600 mg of ursodeoxycholic acid (UDCA) for nearly 6 mo at another clinic. On admission her conjunctiva was neither anemic nor icteric. The laboratory data revealed white blood cell counts of 6 600/μL with a differential of neutrophils 50%, lymphocyte 38%, monocytes 6%, basophils 1%, eosinophils 5%. The C reactive protein level was less than 0.3 mg/dL. The hepatic function profiles showed the total bilirubin to be 0.8 mg/dL, aspartate aminotransferase (AST) 40 IU/L, alanine aminotransferase (ALT) 29 IU/L, lactate dehydrogenase (LDH) 183 IU/L, alkaline phosphatase (ALP) 367 IU/L and gamma-glutamil transpeptidase (γ-GTP) 272 IU/L. Regarding the hepatitis virus, hepatitis B virus surface antigen and hepatitis C virus antibody were both negative. Serology revealed a high level of IgM of 393 mg/dL, and the antinuclear antibody of 80-fold and antimitochondrial antibody (AMA) of 320-fold each and antibody of 176 U/mL were positive for pyruvate dehydrogenase complex-E2. Histopathologically, a damaged bile duct with aggregates of lymphocytes with a nonsuppurative inflammatory destruction of the small bile duct and granuloma was seen in the portal area, which was compatible with those of Scheuer’s stage 1 of primary biliary cirrhosis.
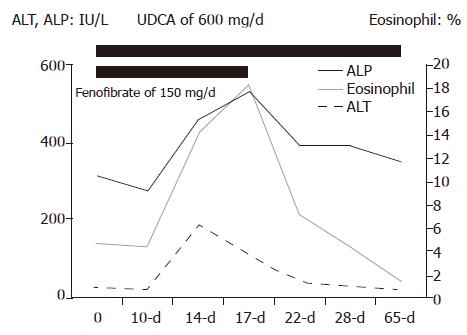
Based on the diagnosis of primary biliary cirrhosis, the administration of fenofibrate 150 mg per day was initiated in addition to 600 mg of UDCA. A fever of over 37.5 °C, anorexia and discomfort in the right hypochondrium appeared 11 d after the administration of fenofibrate. Liver injury such as elevations of total bilirubin of 1.8 mg/dL, AST 268, ALT 216, ALP 537, and γ-GTP 660 IU/L was confirmed, and both the CRP level and the ratio of eosinophils in the peripheral blood increased to 5.14 mg/dL and 14.4 %, respectively. She was diagnosed to have fenofibrate-induced liver injury based on the laboratory data and the clinical course. Therefore, fenofibrate was discontinued, however, UDCA continued to be administered continuously. Thereafter, the serum concentrations of AST, ALT, ALP, γ-GTP, CRP, and the rate of eosinophils rapidly returned to the pretreatment levels of 30, 27, 457, 399 IU/L, less than 0.3 mg/dL and 4.6 %, respectively, 12 d after the discontinuation of fenofibrate (Figure 1). Later, the lymphocyte stimulation index for fenofibrate for this patient was found to be positive, while showing a stimulation index of 212 % in comparison to normal samples.
Several clinical studies on lipoprotein-lowering agents such as simvastatin[3] and bezafibrate[4-7] on PBC patients who failed to respond to UDCA have so far been conducted, and the results have been found to be of value. In addition, fenofibrate, a member of such fibrate class agents as bezafibrate, has also recently been found to be a likely agent for PBC because of its stronger anti-inflammatory effect via PPARα, and its greater ability to reduce the levels of TG and LDL-C than that of bezafibrate. We previously found fenofibrate to effectively treat UDCA-resistant PBC in nine cases without any adverse effect[2].
Regarding the adverse effect of fenofibrate, a Diabetes Arteriosclerosis Intervention Study (DAIS) showed that micronized fenofibrate at 200 mg (equivalent to 300 mg of the standard formulation) was administered for 3 years to type 2 diabetic patients in order to observe the inhibitory effect in the progression of coronary arterial stenosis. As a result, no difference in the safety between fenofibrate and placebo was observed[8]. In addition, studies of human first-generation cultured cells and HepG2 cells suggested the serum aminotransferase levels to be transiently elevated and normalized or returned to pretreatment levels[9]. The increase in the aminotransferase level which occurs after treatment with fenofibrate is not considered to be clinically significant although fenofibrate activates the aminotransferase gene expression, thus leading to a mild and transient elevation of aminotransferase via PPARα through mechanisms involving increased levels of reactive oxygen species and intracellular glutathion depletion, thus leading to mitochondrial dysfunction and a perturbation of intracellular Ca++ homeostasis and also cell death[9].
Since fenofibrate is a widely prescribed therapeutic agent world-wide for patients with hypercholesterolemia and recently for those with PBC[1,2], the early recognition of any possible liver dysfunction especially in the case of PBC, and the immediate cessation of its administration, when positively identified are thus called for to avoid any dangerous clinical complications. Furthermore, our case shows that fenofibrate-induced liver injury might occur in addition to the transient elevations in the AST and ALT levels via PPARα.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2147-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Dohmen K, Mizuta T, Nakamuta M, Shimohashi N, Ishibashi H, Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol. 2004;10:894-898. [PubMed] |
| 3. | Ritzel U, Leonhardt U, Näther M, Schäfer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. Am J Gastroenterol. 2000;95:326-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Miyaguchi S, Ebinuma H, Imaeda H, Nitta Y, Watanabe T, Saito H, Ishii H. A novel treatment for refractory primary biliary cirrhosis? Hepatogastroenterology. 2000;47:1518-1521. [PubMed] |
| 6. | Kurihara T, Niimi A, Maeda A, Shigemoto M, Yamashita K. Bezafibrate in the treatment of primary biliary cirrhosis: comparison with ursodeoxycholic acid. Am J Gastroenterol. 2000;95:2990-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Yano K, Kato H, Morita S, Takahara O, Ishibashi H, Furukawa R. Is bezafibrate histologically effective for primary biliary cirrhosis? Am J Gastroenterol. 2002;97:1075-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Diabetes Arteriosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary–artery disease in type 2 diabetes: the Diabetes Arteriosclerosis Intervention Study, a randomized study. Lancet. 2001;357:905-910. [DOI] [Full Text] |
| 9. | Edgar AD, Tomkiewicz C, Costet P, Legendre C, Aggerbeck M, Bouguet J, Staels B, Guyomard C, Pineau T, Barouki R. Fenofibrate modifies transaminase gene expression via a peroxisome proliferator activated receptor alpha-dependent pathway. Toxicol Lett. 1998;98:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (1)] |