Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7646
Revised: August 1, 2005
Accepted: August 3, 2005
Published online: December 28, 2005
AIM: To analyze the serum levels of retinoids and Leiden mutation in patients with esophageal, gastric, liver, pancreatic, and colorectal cancers.
METHODS: The changes in serum levels of retinoids (vitamin A, α- and β-carotene, α- and β-cryptoxanthin, zeaxanthin, lutein) and Leiden mutation were measured by high liquid performance chromatography (HPLC) and polymerase chain reaction (PCR) in 107 patients (70 males/37 females) with esophageal (0/8), gastric (16/5), liver (8/7), pancreatic (6/4), and colorectal (30/21 including 9 patients suffering from in situ colon cancer) cancer. Fifty-seven healthy subjects (in matched groups) for controls of serum retinoids and 600 healthy blood donors for Leiden mutation were used.
RESULTS: The serum levels of vitamin A and zeaxanthin were decreased significantly in all groups of patients with gastrointestinal (GI) tumors except for vitamin A in patients with pancreatic cancer. No changes were obtained in the serum levels of α- and β-carotene, α- and β-cryptoxanthin, zeaxanthin, lutein in patients with GI cancer. The prevalence of Leiden mutation significantly increased in all groups of patients with GI cancer.
CONCLUSION: Retinoids (as environmental factors) are decreased significantly with increased prevalence of Leiden mutation (as a genetic factor) in patients before the clinical manifestation of histologically different (planocellular and hepatocellular carcinoma, and adenocarcinoma) GI cancer.
- Citation: Mózsik G, Rumi G, Dömötör A, Figler M, Gasztonyi B, Papp E, Pár A, Pár G, Belágyi J, Matus Z, Melegh B. Involvement of serum retinoids and Leiden mutation in patients with esophageal, gastric, liver, pancreatic, and colorectal cancers in Hungary. World J Gastroenterol 2005; 11(48): 7646-7650
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7646.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7646
The number of patients with different gastrointestinal (esophageal, gastric, liver, pancreatic, and colorectal) cancer has increased about two- to threefolds in the last decades (except for gastric cancer which has decreased 50%) in Hungary. The number of patients who died of these malignant diseases represents the second highest population of the total mortality of patients in Hungary. The second highest mortality rate of gastrointestinal (GI) tumor takes place at the second place in our country.
The causes of GI tumors are not known. However, different genetic and environmental factors play a role in the development of GI cancer. It is generally accepted that different diseases such as acute and chronic inflammatory diseases, polyposis, can be taken as precancerous states of GI cancer.
Since the year of l980, we have studied the role of retinoids in protecting gastrointestinal mucosa in animal experiments and human observations[1-4]. Retinoids are chemical compounds of color materials from plants and are built up from C-20 and four isoprene units, while carotenoids are built up from C-40 and eight isoprene units located in about 600 plants, and about 50 from 600 isolated compounds are precursors of vitamin A.
Vitamin A and β-carotene (and other retinoids) have gastric mucosal protective effects in rats provoked by intragastric administration of 1 mL from 0.6 mol/L HCl, 25% NaCl, 0.2 NaOH, and 960 mL/L ethanol, without the presence of any inhibition of gastric acid secretion[1]. Vitamin A has a higher ulcer healing effect than atropine, cimetidine DE-NOL (tripotassio–dicitrato) in patients with gastric ulcer[2-4] .The serum level of retinoids is decreased in patients with inflammatory bowel disease[5-8]. It was also demonstrated that the serum levels of vitamin A and zeaxanthin are also decreased in patients with GI cancer[9], but the possible correlation between the changes in other serum retinoids and the prevalence of Leiden mutation has not been studied.
The presence of Leiden mutation (replacement of Arg by Glu of residue 506 in the factor V molecule, FVR, 506 Q) has been proven in thrombophylia[10-13] as well as in Crohn’s disease and ulcerative colitis[14-18], meanwhile no higher prevalence of Leiden mutation has been obtained in patients with acute gastritis and hepatitis[17]. The significant presence of Leiden mutation (APC) is responsible for blood coagulation abnormality in thrombophylia.
The aims of our present study were to evaluate the changes in serum levels of retinoids (as nutritional components of vitamin A, β-carotene, α-carotene, α- and β-cryptoxanthin, zeaxanthin, lutein) in patients with different gastrointestinal (esophageal, gastric, liver, pancreatic, and colorectal) cancer, to study the prevalence of Leiden mutation in the above mentioned patients, to find some correlation between the changes in Leiden mutation and serum level of vitamin A and zeaxanthin in GI cancer patients as well as between GI cancer development and chemical structure of retinoids, to obtain some correlation between serum levels of vitamin A and zeaxanthin in patients with different GI tumors.
Observations were carried in 107 patients with esophageal (n = 8), gastric (n = 21), liver (n = 15), pancreatic (n = 10), colorectal (n = 53), and in situ colon (n = 9) cancer, including 70 males (50±12 years) and 37 females (49±10 years). Fifty-seven healthy persons (in matched group) were used as control, and 600 healthy blood donors were used for the control of Leiden mutation (Table 1). A total of 764 patients with gastrointestinal cancer and healthy subjects were included in this study. The studies were approved by the Ethical Committee of University of Pécs, Hungary. Written informed consent was obtained from all participants and the nature of the study was fully explained (Table 1).
| Number of patients | ||||
| Types of tumors | Male | Female | Total | Histology |
| Esophageal | 8 (60±10 yr)1 | – | 8 | Planocellular carcinoma |
| Gastric | 16 (64±12 yr)1 | 5 (68±10 yr)1 | 21 | Adenocarcinoma |
| Liver | 8 (60±8 yr)1 | 7 (57±13 yr)1 | 15 | Hepatocellular carcinoma |
| Pancreas | 6 (56±11 yr)1 | 4 (63±9 yr)1 | 10 | Adenocarcinoma |
| Colorectal | 30 (66±10 yr)1 | 23 (65±11 yr)1 | 53 | Adenocarcinoma |
| In situ carcinoma in colon polyps | 4 (60±5 yr)1 | 5 (61±5 yr)1 | 9 | Adenocarcinoma |
| Total | 70 | 37 | 107 | |
| Healthy subjects | 29 (50±12 yr)1 | 28 (49±10 yr)1 | 57 |
Physical, laboratory, iconographic, and histological examinations were carried out in the patients with gastrointestinal tumor. The diagnostic histology indicated planocellular carcinoma in esophagus, hepatocellular carcinoma in liver and adenocarcinoma in stomach, pancreas and colorectum. The control (healthy subjects) persons received physical and laboratory screening, and the medical histories were found to be negative (including the genetic backgrounds).
The serum levels of retinoids were measured by high performance liquid chromatography (HPLC). The serum levels of vitamin A, α- and β-carotene, α- and β-cryptoxanthin, zeaxanthin and lutein both in the control (healthy subjects) and in patients with GI tumors were measured. Blood samples were prepared for HPLC measurements: 2 mL of serum sample was shaken with
2 mL/L ethanol for 2 min, and extracted with 3 mL hexane for 2 min. The mixture was centrifuged for 5 min. As an internal standard, canthaxanthin was added to the removed homogenous organic phase, evaporated in vacuum. The residue was dissolved in 0.2 mL 1:4 dichloromethane/methanol and 0.125 mL of this solution was injected. The chromatographic system consists of a gradient former Model 250 B Glenco injector (Glycotec, Germany) and a time programmable UV-vis detector Model 166-2, equipped with Gold chromatography software (Beckmann, USA).The column is 150 mm×4.6 mm in size packed with Chromsil-C 0.186 mm. The eluent was 30 mL/L water in methanol (A), methanol (B) and 20 v/v dichloromethane in methanol. The flow rate was 1.5 mL/min. The gradient program was 100% A for 30 s, 100% B for 3 min, to 100% for 4 min (linear steps). The time program of wavelength was 323 mm for 3.5 min (detecting vitamin A), then 450 mm (detecting other retinoids). The chromatograms were evaluated quantitatively by relating the peak areas of the individual components to canthaxanthin used as internal standard. The ratio of the molar extinctions of the authentic samples to that of canthaxanthin was employed as a correction factor of the detector signals. The results were given in μmol/L, and expressed as mean±SE.
Leiden mutation was detected by polymerase-chain reaction (PCR)[12]. The DNA was isolated from 3 mL EDTA blood.
The changes in serum levels of retinoids were detected by the method of ANOVA. The prevalence of Leiden mutation was statistically analyzed by χ2 test. P<0.05 (in the changes of serum retinoids and prevalence of Leiden mutation) was considered statistically significant.
The serum levels of vitamin A were decreased in all groups of patients with esophageal, gastric, hepatocellular and colorectal cancer meanwhile its level remained normal in patients with pancreatic cancer. The serum levels of α- and β-carotene, as provitamins of vitamin A, were normal in different groups of patients with GI cancer. Zeaxanthin level (without presence of any vitamin A property) was decreased significantly in patients with esophageal, gastric, hepatocellular, pancreatic, and colorectal cancer. No changes were obtained in the serum levels of α- and β-cryptoxanthin and lutein in the studied cancer patients (Table 2).
| Retinoids | Healthy | Esophageal | Gastric | Liver | Pancreatic | Colon | In situ colon |
| subjects | cancer | cancer | cancer | cancer | cancer | cancer | |
| Vitamin A | 2.07±0.12 | 0.14±0.04b | 1.02±0.10b | 0.75±0.07c | 1.68±0.10NS | 0.35±0.02c | 0.30±002c |
| α-Carotene | 3.93±0.40 | 3.81±0.50NS | 3.85±0.60NS | 3.82±0.50NS | 3.90±0.40NS | 3.80±0.70NS | 3.80±0.70NS |
| β-Carotene | 8.59±0.40 | 7.50±0.30NS | 8.01±0.35NS | 8.10±0.30NS | 8.40±0.40NS | 6.80±0.40NS | 7.90±0.30NS |
| α-Cryptoxanthin | 4.10±0.50 | 4.00±0.60NS | 3.90±0.50NS | 4.00±0.40NS | 3.90±0.40NS | 4.00±0.30NS | 4.00±0.30NS |
| β-Cryptoxanthin | 6.00±0.60 | 5.90±0.40NS | 6.00±0.50NS | 5.90±0.40NS | 5.90±0.50NS | 4.95±0.40NS | 4.90±0.30NS |
| Zeaxanthin | 0.14±0.01 | 0.074±0.007b | 0.08±0.004a | 0.05±0.005c | 0.03±0.002c | 0.07±0.004c | 0.03±0.002c |
| Lutein | 0.11±0.007 | 0.10±0.04NS | 0.10±0.02NS | 0.08±0.007NS | 0.06±0.004b | 0.010±0.04NS | 0.10±0.04NS |
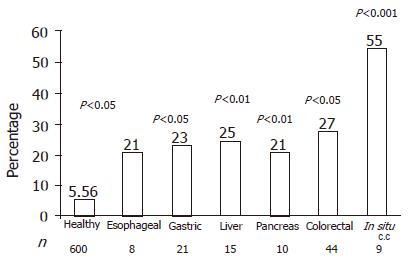
Prevalence of Leiden mutation accounted for 5.56% in 600 healthy blood donors, which was significantly higher in patients with esophageal (P<0.05), gastric (P<0.01), liver (P<0.01), pancreatic (P<0.05), colorectal (P<0.001) cancer. The higher prevalence of Leiden mutation (55%) was found in situ colorectal cancer (P<0.001, Figures 1 and 2).
Retinoids are chemical compounds of color materials from plants. Increased intake of plant foods can prevent different types of GI cancer. We studied the possible role of different retinoids (vitamin A, α- and β-carotene, α- and β-cryptoxanthin, zeaxanthin, and lutein) in patients with different GI cancer based on previous studies[5-9,19,20]. The location of GI tumor differed in organs (esophagus, stomach, pancreas, liver, and colon), suggesting that different etiological factors are involved in the development of different GI cancer (Barrett’s esophageal metaplasia, chronic atrophic gastritis, viral infection in liver, chronic inflammatory bowel disease) in our everyday medical practice.
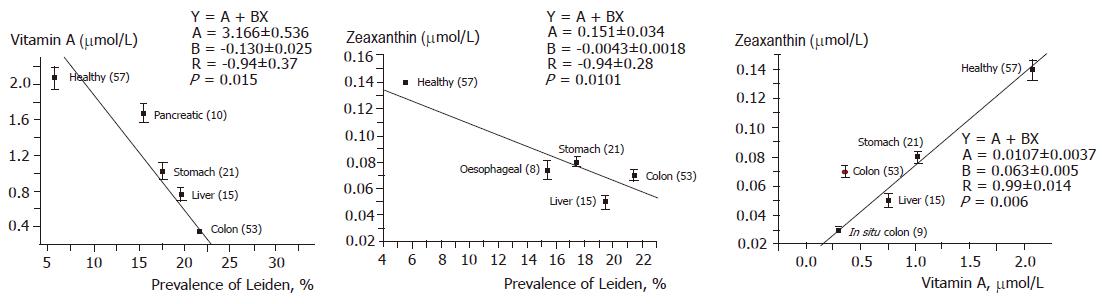
The serum levels of vitamin A and zeaxanthin were decreased significantly in all groups of GI cancer patients (not in patients with pancreatic cancer). Surprisingly the serum levels of provitamins were normal in patients with different GI tumor. These results indicate that transformation of provitamins into vitamin A is impaired by some factors at the level of liver, suggesting that the liver plays a key role in the development of tumor. Similar changes were observed in the serum levels of retinoids in patients with hepatocellular cancer, which offers a further proof for this hypothesis.
It is also interesting to evaluate the possible correlation between the terminal chemical structure, vitamin A activity and GI mucosal protection. Our results have clearly proved that there is no close correlation between the terminal chemical structure, vitamin A activity and GI mucosal protection (Table 3).
| Esophageal cancer | Gastric cancer | Hepatocellular cancer | Pancreatic cancer | Colorectal cancer | In situ colon cancer | |
| Patients | 8 | 21 | 15 | 10 | 44 | 9 |
| Vitamin A | ↓↓ | ↓↓ | ↓↓↓ | NS | ↓↓↓ | ↓↓↓ |
| a-Carotene | NS | NS | NS | NS | NS | NS |
| b-Carotene | NS | NS | NS | NS | NS | NS |
| a-Cryptoxantin | NS | NS | NS | NS | NS | NS |
| b-Cryptoxantin | NS | NS | NS | NS | NS | NS |
| Zeaxantin | ↓↓ | ↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| Lutein | NS | NS | NS | ↓↓ | NS | NS |
Similar results have been obtained in animal experiments[1,21-24] (Table 4). At present, no information is available on the correlation between the serum and tissue levels of retinoids in patients with different GI tumor. These observations cannot be done due to the obligatory necessity of histological evaluation of tumor tissues.
| Retinoids | Terminal chemicalstructure | Vitamin Aactivity | Gastric mucosalprevention |
| Vitamin A | R = a | Yes | Yes |
| β-Carotene | X = Y = a | Yes | Yes |
| β-Cryptoxanthin | X = a, Y = b | Yes | Yes |
| Zeaxanthin | X = Y = b | None | Yes |
| Lutein | X = b, Y = c | None | None |
| Capsorubin | X = Y = d | None | None |
| Capsanthin | X = b, Y = d | None | None |
| Capsanthol | X = b, Y = e | None | None |
| Lycopene | X = Y = f | None | None |
In animal experiments, β-carotene has been found in gastric mucosa of indomethacin-treated rats after acute surgical vagatomy[25,26], however no gastric mucosal protection is found, indicating that intact vagal nerve is necessary for the development of β-carotene-induced gastric cytoprotection[26]. The mechanism of retinoids is very complex. Our earlier observations indicate that the GI mucosal protective effect of retinoids depends on intact vagal nerve and adrenals as well as on gastric mucosal biochemical changes (retinoids produce a dose-dependent inhibition on the extent of ATP-transformation into ADP in association with a simultaneous increase in the transformation of ATP into cAMP), intact function of sulfhydryl groups and scavenger properties[9,19-28].
Retinoid-induced GI mucosal protection does not depend on the inhibition of gastric acid secretory responses, vitamin A activity, number of unsaturated double bonds, presence of a characteristic chemical structure of their terminal components and modification of vascular permeability[20]. These results clearly indicate that the beneficial effect of retinoids is much more complex than that of their scavengers. The results of biochemical observations suggest that different cAMP-dependent cellular regulatory mechanisms exist (including the functions of retinoid receptors, gene expressions)[7,20,27].
The involvement of vascular events is suggested in the development of different acute inflammatory processes in the GI tract[20]. That is the reason why we studied the potential role of Leiden mutation in acute and chronic gastrointestinal inflammatory processes (Helicobacter pylori-induced gastritis, viral hepatitis, Crohn’s disease, ulcerative colitis). The prevalence of Leiden mutation increased in chronic inflammatory bowel diseases, but no changes were obtained in gastritis and hepatitis. The prevalence of Leiden mutation was also significantly higher in patients with esophageal, gastric, hepatocellular, pancreatic, and colorectal cancer. These results indicate that only the increased prevalence of Leiden mutation does not take place directly in the tumor genesis of human GI cancer. We compared the different results in the examined parameters in patients with acute and chronic gastrointestinal inflammatory diseases, and found a time-sequence process between the inflammatory diseases and GI cancer in patients, suggesting that retinoids play a key role in the development of precancerous state to cancerous state[5,8,20].
In conclusion, the prevalence of Leiden mutation is significantly correlated with decrease in serum levels of vitamin A and zeaxanthin, suggesting that retinoids play a role in the human GI tumor genesis.
The authors express their thanks to Ms. Katalin Vincze and Ms. Erika Kispap for the careful preparation of the manuscript.
| 1. | Jávor T, Bata M, Lovász L, Morón F, Nagy L, Patty I, Szabolcs J, Tárnok F, Tóth G, Mózsik G. Gastric cytoprotective effects of vitamin A and other carotenoids. Int J Tissue React. 1983;5:289-296. [PubMed] |
| 2. | Patty I, Benedek S, Deák G, Jávor T, Kenéz P, Nagy L, Simon L, Tárnok F, Mózsik G. Controlled trial of vitamin A therapy in gastric ulcer. Lancet. 1982;2:876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Patty I, Benedek S, Deák G, Jávor T, Kenéz P, Morón F, Nagy L, Simon L, Tárnok F, Mózsik G. Cytoprotective effect of vitamin A and its clinical importance in the treatment of patients with chronic gastric ulcer. Int J Tissue React. 1983;5:301-307. [PubMed] |
| 4. | Patty I, Tárnok F, Simon L, Jávor T, Deák G, Benedek S, Kenéz P, Nagy L, Mózsik G. A comparative dynamic study of the effectiveness of gastric cytoprotection by vitamin A, De-Nol, sucralfate and ulcer healing by pirenzepine in patients with chronic gastric ulcer (a multiclinical and randomized study). Acta Physiol Hung. 1984;64:379-384. [PubMed] |
| 5. | Rumi G, Szabó I, Vincze A, Matus Z, Tóth G, Rumi G, Mózsik G. Decrease in serum levels of vitamin A and zeaxanthin in patients with colorectal polyp. Eur J Gastroenterol Hepatol. 1999;11:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Rumi G, Szabó I, Vincze A, Matus Z, Tóth G, Mózsik G. Decrease of serum carotenoids in Crohn's disease. J Physiol Paris. 2000;94:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Mózsik G, Bódis B, Karádi O, Király A, Nagy L, Rumi G, Süto G, Szabó I, Vincze A. Cellular mechanisms of beta-carotene-induced gastric cytoprotection in indomethacin-treated rats. Inflammopharmacology. 1998;6:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Mózsik G, Nagy Z, Nagy A, Rumi G, Karádi O, Czimmer J, Matus Z, Tóth G, Pár A. Leiden mutation (as genetic) and environmental (retinoids) sequences in the acute and chronic inflammatory and premalignant colon disease in human gastrointestinal tract. J Physiol Paris. 2001;95:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Rumi Gy, Pár A, Matus Z, Rumi Gy, Mózsik Gy The Defensive Effects of Retinoids in the Gastrointestinal Tract (Animal Experiments and Human Observations). Budapest, Akadémiai Kiadó. 2001;1-79. |
| 10. | Bargen JA, Barker NW . Extensive arterial and venous thrombosis complicating chronic ulcerative colitis. Arch Intern Med. 1936;58:17-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Dahlbäck B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci USA. 1993;90:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1264] [Cited by in RCA: 1144] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 12. | Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2476] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 13. | Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost. 1995;74:139-148. [PubMed] |
| 14. | Talbot RW, Heppell J, Dozois RR, Beart RW. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 412] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 16. | Nagy Z, Nagy A, Karádi O, Pár A, Mózsik G. The high prevalence of the factor V Leiden mutation in central European inflammatory bowel disease patients. Am J Gastroenterol. 2000;95:3013-3014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Nagy Z, Nagy A, Karádi O, Figler M, Rumi G, Süto G, Vincze A, Pár A, Mózsik G. Prevalence of the factor V Leiden mutation in human inflammatory bowel disease with different activity. J Physiol Paris. 2001;95:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Papa A, Danese S, Grillo A, Gasbarrini G, Gasbarrini A. Review article: inherited thrombophilia in inflammatory bowel disease. Am J Gastroenterol. 2003;98:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Mózsik Gy, Pár A, Pár G, Gasztonyi B, Figler M Nutritional gastrointestinal mucosal protection: an update overview. In: Sikiric P, Seiwerth S., Mózsik Gy., Arakava T., Takeuchi K., Ulcer Research. Bologna Monduzzi Editore. 1994;155-162. |
| 20. | Mózsik Gy, Neural, hormonal and pharmacological regulations of retinoids-induced gastrointestinal musocal protection. Recent Res Develop in Life Sci. 2005;3:131-202. |
| 21. | Mózsik Gy, Garamszegi M, Jávor T, Sütő G, Vincze Á, Tóth Gy, Zsoldos T Correlations between the oxygen free radicals, membrane-bound ATP-dependent energy systems in relation of development of ethanol- and HCl- induced gastric mucosal damage and of β-carotene-induced gastric cytorprotection. In: Tsuchiya M, Kawai K, Kondo M, Yoshikawa T, eds. Free Radicals in Digestive Diseases. Amsterdam: Elsevier Science Publisher Co., Inc. 1988;111-116. |
| 22. | Mózsik Gy, Figler M, Garamszegi M, Jávor T, Nagy L, Sütő G, Vincze Á, Zsoldos T Mechanism of gastric mucosal cytoprotection. I. Time-sequence analysis of gastric mucosal membrane-bound ATP-dependent energy systems, oxygen free radicals and macroscopically appearance of gastric cytoprotection by PGI2 and β-carotene in HCL- model of rats. In: Hayashi E, Niki M, Kondo M, Yoshikawa T eds. Medical, Biochemical, and Chemical Aspects of Free Radicals. Amsterdam: Elsevier Science Publishers Co, Inc. 1989;1421-1425. |
| 23. | Mózsik Gy, Figler M, Garamszegi M, Jávor T, Nagy L, Sütő G, Vincze Á, Zsoldos T Mechanism of gastric mucosal cytoprotection. II. Time-sequence analysis of gastric mucosal membrane-bound ATP-dependent energy systems, oxygen free radicals and appearance of gastric mucosal damage. In: Hayashi E, Niki M, Kondo M, Yoshikawa T eds. Medical, Biochemical, and Chemical Aspects of Free Radicals. Amsterdam Elsevier Science Publishers, Co, Inc. 1989;1427-1431. |
| 24. | Mózsik Gy, Jávor T. Therapy of ulcers with sulfhydryl and nonsulfhydryl antioxidants. In: Swabb A, Szabo S eds. Ulcer Disease. Investigation and Basis for Therapy. New York, Basel, Hong Kong Marcel Dekker Inc. 1991;321-341. |
| 25. | Mózsik G, Király A, Garamszegi M, Jávor T, Nagy L, Sütó G, Tóth G, Vincze A. Failure of prostacyclin, beta-carotene, atropine and cimetidine to produce gastric cyto- and general mucosal protection in surgically vagotomized rats. Life Sci. 1991;49:1383-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Mózsik G, Nagy Z, Nagy A, Rumi G, Karádi O, Czimmer J, Matus Z, Tóth G, Pár A. Leiden mutation (as genetic) and environmental (retinoids) sequences in the acute and chronic inflammatory and premalignant colon disease in human gastrointestinal tract. J Physiol Paris. 2001;95:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Mózsik G, Bódis B, Figler M, Király A, Karádi O, Pár A, Rumi G, Sütõ G, Tóth G, Vincze A. Mechanisms of action of retinoids in gastrointestinal mucosal protection in animals, human healthy subjects and patients. Life Sci. 2001;69:3103-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Mózsik Gy, Bódis B, Garamszegi M, Karádi O, Király Á, Nagy L, Sütő G, Tóth Gy, Vincze Á Role of vagal nerve in the development of gastric mucosal injury and its prevention by atropine, cimetidine, β-carotene and prostacyclin in rats. In: Szabo S, Tache Y, Neuroendocrinology of Gastrointestinal Ulceration. New York Plenum Press. 1995;175-190. |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK