Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7602
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: December 28, 2005
AIM: To investigate the effect of cyclosporine A (CsA), FK-506, and mycophenolate mofetil (MMF) and 40-0-[2-hydroxyethyl]rapamycin (RAD) on proliferation of human intrahepatic biliary epithelial cells (BECs) in vitro.
METHODS: BECs were isolated from six human liver tissuespecimens with the immunomagnetic separation method and treated with different concentrations of CsA, FK-506, RAD, and MMF in vitro. Proliferation of the cells was measured by MTT assay at 24 and 48 h after treatment, respectively. One-way analysis of variance was used to analyze the results. Expression of CK 19 in BECs was monitored by flow cytometry and Western blot.
RESULTS: Six lines of BECs were established. They survived for 4-18 wk in vitro. Flow cytometry analysis showed that these cells always expressed CK19. CsA, FK-506, RAD, and MMF inhibited proliferation of BECs in a dose-dependent manner. The lowest concentration of CsA, FK-506, RAD, and MMF to inhibit proliferation of BECs (P<0.05) was 500, 100, 0.25, and 100 µg/L, respectively. However, the expression of CK19 by BECs was not changed.
CONCLUSION: CsA, FK-506, RAD, and MMF have an antiproliferative effect on human intrahepatic BECs in vitro, while RAD has the strongest growth-inhibitory effect. Their possible effects on liver regeneration and bile duct injury in transplant patients should be further investigated.
- Citation: Liu C, Schreiter T, Frilling A, Dahmen U, Broelsch CE, Gerken G, Treichel U. Cyclosporine A, FK-506, 40-0-[2-hydroxyethyl]rapamycin and mycophenolate mofetil inhibit proliferation of human intrahepatic biliary epithelial cells in vitro. World J Gastroenterol 2005; 11(48): 7602-7606
- URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7602
Within the last decade, living donor liver transplantation (LDLT) has become an accepted treatment for adult patients with end-staged liver disease and it provides a viable alternative to many recipients who would otherwise die while on the waiting lists. Part of the donor liver used for adult LDLT is small in size when transplanted into the recipient. The liver has the unique ability to regenerate and adjust its size according to the requirement of the recipient. Therefore, liver regeneration is crucial for a successful LDLT. The volume and quality of the graft, ischemic time, portal flow, venous outflow, and immunosuppressive agents are among the factors affecting liver regeneration after adult LDLT.
Cyclosporine A (CsA), FK-506, and MMF can augment liver regeneration after hepatectomy or orthotopic liver transplantation in rats[1-6]. However, in vitro experiments indicate that CsA and FK-506 have an antiproliferative or cytotoxic effect on adult human hepatocytes[7,8]. During liver regeneration after partial hepatectomy, both mature hepatocytes and BECs are induced to proliferate. Human hepatocytes and intrahepatic BECs can be isolated from the liver tissue and maintained in vitro for a certain time. In vitro, mature hepatocytes have a very limited ability to replicate, while BECs have a great potential to proliferate[9,10]. In this study, the effect of CsA, FK-506, MMF, and 40-0-[2-hydroxyethyl]rapamycin (RAD) on the proliferation of human intrahepatic BECs in vitro was investigated.
All liver specimens were taken from the patients who underwent hepatectomy or liver transplantation at University Hospital Essen, Germany. The procedure confirmed to the local ethical guidelines and followed the ethical guidelines of the 1975 Declaration of Helsinki. All patients or their relatives gave informed consents. Three diseased liver tissues were obtained from explanted livers, including one case of primary biliary cirrhosis (64 years old), one case of oxalosis (one year old) and one case of carbamoyl-phosphate-synthetase deficiency (one year old). Two normal liver tissues were obtained during hepatectomy, including one case of hepatoblastoma (1.5 years old) and one case of hepatocellular carcinoma (67 years old). Another normal liver tissue was obtained from a donor (10 years old) when the graft exceeded surgical requirement. All tissues were stored in Dulbecco’s minimum essential medium (PAA Laboratories, Coelbe, Germany) at 4 ºC and used within 36 h after hepatectomy.
Human intrahepatic BECs were isolated with an immunomagnetic separation method employing an antibody against human epithelial antigen (HEA) as reported[11,12]. BECs were cultured with the plating medium in a 25-cm2 flask (Greiner, Frickenhausen, Germany) pre-coated with collagen G (Biochrom KG, Berlin). The cell culture was maintained in a humidified atmosphere composed of 95% air and 50 mL/L CO2. About 24-72 h after plating, the growth medium containing hepatocyte growth factor (HGF, R&D systems, UK) was used to culture the cells. The composition of the plating medium and growth medium was similar to the reported[13]. At confluence, BECs were detached with a solution containing 0.1 g/L trypsin and 1.0 g/L EDTA (Sigma, Taufkirchen, Germany) and maintained in a culture or used for the experiment.
The expression of CK19 by BECs was investigated using flow cytometry with a FACS-Scan machine (Becton Dickinson, Heidelberg, Germany). Cells were pre-treated with the Fix & Perm cell permeabilization kit (Dianova, Hamburg, Germany). The monoclonal antibody against CK19 (IgG2b, Progen, Heidelberg, Germany) was used in a three times dilution according to the manufacturer’s instructions. The FITC-labeled second antibody (Dianova, Hamburg, Germany) was diluted fifty times.
During passage, BECs were seeded at the density of 1×104 viable cells/well in 24-well plates with the plating medium. One day later, the medium was changed to the growth medium containing the drugs. CsA (MW 1203, Sandoz, Switzerland), FK 506 (MW 822, Fujisawa, Japan), RAD (MW 958.25, Sandoz, Switzerland) and MMF (MW 433.5, Sandoz, Switzerland) were diluted with a phosphate-buffered solution (PBS, PAA Laboratories, Coelbe, Germany). The concentrations of CsA, FK 506, RAD, and MMF in the medium ranged from 10 to 1 000 µg/L, 10 to 1 000 µg/L, 0.05 to 10 µg/L, 0.05 to 500 µg/L, respectively. Each concentration of the drug was determined in eight wells per experiment. The vigorously proliferating BECs derived from each liver tissue were used in the study and each drug was investigated eight times. Negative control cultures only received the growth medium.
Dimethylthiazol-diphenyl-tetrazolium (MTT) assay was performed at 24 and 48 h after addition of the drugs, respectively. Fifty microliters of 4 mg/mL MTT (Sigma, Taufkirchen, Germany) was given to each well (containing 0.5 mL medium) in the 24-well plate. Four hours later, the medium was removed and 250 µL dimethyl sulfoxide (DMSO) was added into each well to solubilize the formazan dye converted from MTT by the cells. Five minutes later, the plates were shaken to make a homogenous dye solution and measured immediately with an ELISA reader (Lambda E, MWG-Biotech, Ebersberg, Germany) at a wavelength of 550 nm minus 690 nm. The cell number in each well was recorded in the form of optical density (OD).
BECs were passaged into six-well plates pre-coated with collagen G. After 24 h, CsA or FK-506 was added into the growth medium and the cells were cultured for 48 h. The concentrations of both CsA and FK-506 in the medium were 500, 1 000 and 2 000 µg/L, respectively. BECs were detached by the use of 0.05% collagenase XI (Sigma, Taufkirchen, Germany), washed with PBS and stored at –20 °C. Protein amount was determined with the Bradford method (Biorad, Munich, Germany) after the cell pellet was boiled 10 times with 40 µL of 0.1% Triton/PBS. Extracted protein (1 µg/lane) was electrophoretically separated on a 10% SDS-PAGE and transferred to nitrocellulose. For the staining, blots were blocked with 1.2% gelatin in PBS and washed with 0.05% Tween 20 in PBS. First antibody against CK19 (IgG2b, Progen, Heidelberg, Germany) was diluted 1:80 in PBS and incubated for 1 h at room temperature. Anti-mouse Ig AP-conjugate as second antibody was diluted 1:1 000 in PBS and also incubated for 1 h at room temperature. Finally, the color was developed using BCIP and NBT (Gerbu, Gaiberg, Germany).
The average OD measured at different concentrations of each drug was compared using one-way analysis of variance (ANOVA) with the software SPSS 11.0 (SPSS, Inc., Chicago, IL, USA). Proliferation of the cells exposed to the drugs was compared to the negative control. P<0.05 was considered statistically significant.
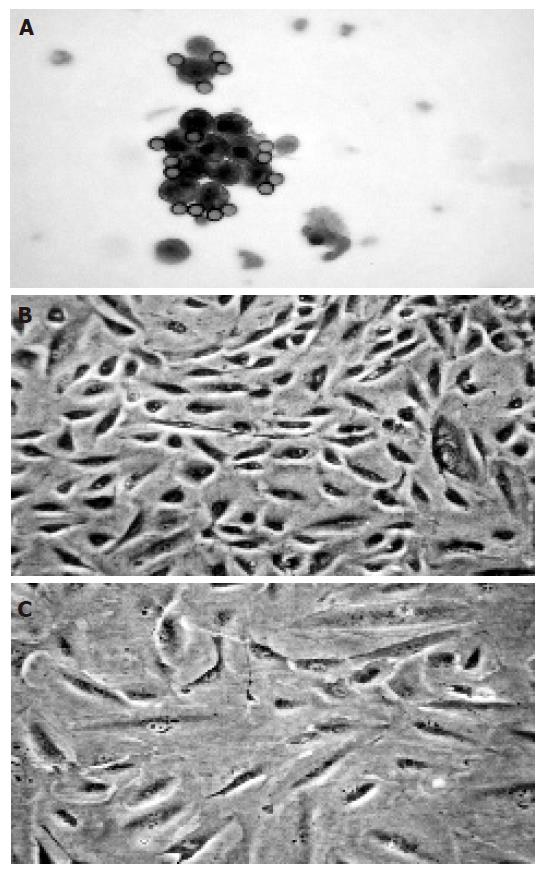
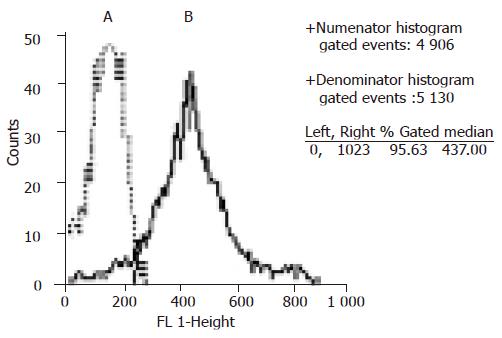
The freshly isolated cells were small and round in shape and most of them formed clusters. Magnetic beads could be seen on the cell surface (Figure 1A). Intrahepatic BECs isolated from six liver tissues proliferated for 4-18 wk in vitro and were passaged 3-12 times. The proliferating BECs were small and oval in shape (Figure 1B). Flow cytometry analysis showed that the proliferating BECs consistently expressed CK19 (Figure 2).
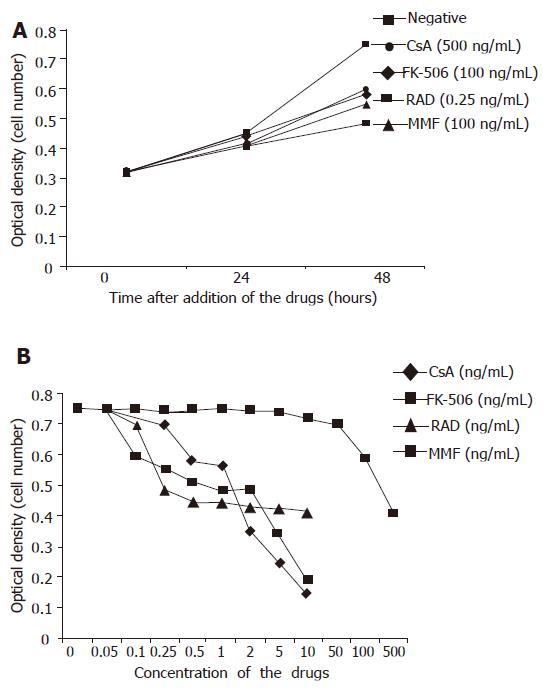
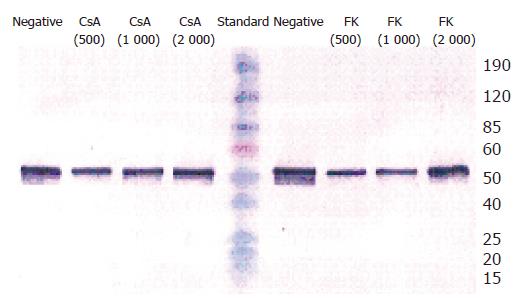
The vigorously proliferating BECs which represented passage (P) 1–6 BECs derived from different liver tissues, were exposed to the drugs. In this set of experiments, all the four immunosuppressive agents had no growth-promoting effect on BECs at any concentration in vitro. On the contrary, they inhibited the proliferation of BECs and this could be clearly shown at 48 h after the addition of the drugs (Figure 3A). All the four drugs inhibited the proliferation of BECs in a dose-dependent manner (Figure 3B) and this was repeatedly demonstrated with BECs derived from different liver tissues and in different passages. The lowest concentration for CsA, FK-506, RAD, and MMF to inhibit the proliferation of BECs (P<0.05) was 500, 100, 0.25, and 100 µg/L, respectively (Figures 3A and B). RAD had the strongest growth-inhibiting effect. Microscopically, the BECs treated with the drugs became larger than the cells in negative control (Figure 1C). However, Western blot analysis showed that CsA and FK-506 did not change CK19 protein expression by BECs (Figure 4).
Intrahepatic BECs represent about 5% of total cell population in the liver. In the human liver, human epithelial antigen (HEA) is the cell surface antigen that only exists in intrahepatic BECs. With the immunomagnetic isolation technique employing anti-HEA monoclonal antibody, intrahepatic BECs can be purified after density-gradient centrifugation[11]. The purity of isolated human intrahepatic BECs is more than 99%[12]. The specific markers for biliary cells include CK7 and CK19. All the cells used in this experiment expressed CK19 (Figure 2), indicating that they are intrahepatic BECs.
In liver transplantation, the damaged liver cells caused by ischemia-reperfusion injury are repaired by liver regeneration. In adult LDLT, liver regeneration is of particular importance to meet the necessary metabolism requirement of the recipient. An immunosuppressive agent is one of the factors affecting liver regeneration after liver transplantation.
Generally speaking, CsA and FK-506 exert their immunosuppressive effect by blocking the production of interleukin-2 (IL-2) by T helper cells, while MMF and RAD exert their immunosuppressive effect by inhibiting proliferation of lymphocytes. CsA, FK-506, and MMF can enhance liver regeneration after hepatectomy or small-for-size liver transplantation in animals. The augmented liver regeneration might be an indirect effect, as CsA and FK-506 have anti-proliferative or cytotoxic effect on hepatocytes[7,8]. The possible mechanism for CsA and FK-506 on enhanced liver regeneration is through inhibition of production of IL-2 production and activation of natural killer (NK) cells[6]. The mechanism of MMF underlying accelerated liver regeneration is not fully understood.
In this study, all the four immunosuppressive agents inhibited human intrahepatic BECs in a dose-dependent manner in vitro, indicating that they have a direct inhibiting effect on liver regeneration and this direct effect should not be neglected, especially in adult LDLT. Our results showed that RAD had the strongest inhibiting effect on human intrahepatic BECs in vitro. The minimal concentration of RAD to inhibit proliferation of BECs (0.25 µg/L) in vitro was much lower than that of target serum through RAD level in patients undergoing immunosuppression (8-12 µg/L) after liver transplantation. Though the results obtained from an in vitro experiment were not identical to the results in vivo, it may indicate the importance of the direct effect of RAD on liver regeneration. Also, as an immunosuppressive agent, RAD has been reported to attenuate liver regeneration in a rat hepatectomy model[14]. Moreover, immunosuppressant-induced bile duct damage has been reported in azathioprine[15-18]. RAD and azathioprine share the similar immunosuppressive mechanism and it should be alerted that RAD might also cause bile duct damage in transplant patients.
In conclusion, CsA, FK-506, RAD, and MMF have an anti-proliferative effect on human intrahepatic BECs in vitro, while RAD has the strongest growth-inhibitory effect. Their possible effects on liver regeneration and bile duct injury in transplant patients should be further investigated.
| 1. | Motale P, Mall A, Spearman CW, Lotz Z, Tyler M, Shepherd E, Kahn D. The effect of mycophenolate mofetil on liver regeneration. Transplant Proc. 2001;33:1054-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Mazzaferro V, Porter KA, Scotti-Foglieni CL, Venkataramanan R, Makowka L, Rossaro L, Francavilla A, Todo S, Van Thiel DH, Starzl TE. The hepatotropic influence of cyclosporine. Surgery. 1990;107:533-539. [PubMed] |
| 3. | Starzl TE, Porter KA, Mazzaferro V, Todo S, Fung J, Francavilla A. Hepatotrophic effects of FK506 in dogs. Transplantation. 1991;51:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Kikuchi N, Yamaguchi Y, Mori K, Takata N, Goto M, Makino Y, Hamaguchi H, Hisama N, Ogawa M. Effect of cyclosporine on liver regeneration after orthotopic reduced-size hepatic transplantation in the rat. Dig Dis Sci. 1993;38:1492-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kahn D, Makowka L, Lai H, Eagon PK, Dindzans V, Starzl TE, Van Thiel DH. Cyclosporine augments hepatic regenerative response in rats. Dig Dis Sci. 1990;35:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Francavilla A, Barone M, Todo S, Zeng Q, Porter KA, Starzl TE. Augmentation of rat liver regeneration by FK 506 compared with cyclosporin. Lancet. 1989;2:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Loréal O, Fautrel A, Meunier B, Guyomard C, Guillouzo A, Launois B. FK 506 is less cytotoxic than cyclosporine to human and rat hepatocytes in vitro. Transplant Proc. 1991;23:2825-2828. [PubMed] |
| 8. | Blanc P, Etienne H, Daujat M, Fabre I, Pichard L, Domergue J, Joyeux H, Fourtanier G, Maurel P. Antiproliferative effect of FK 506 and cyclosporine on adult human hepatocytes in culture. Transplant Proc. 1991;23:2821-2824. [PubMed] |
| 9. | Strain AJ. Isolated hepatocytes: use in experimental and clinical hepatology. Gut. 1994;35:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Joplin R. Isolation and culture of biliary epithelial cells. Gut. 1994;35:875-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Joplin R, Strain AJ, Neuberger JM. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell Dev Biol. 1989;25:1189-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Joplin R, Strain AJ, Neuberger JM. Biliary epithelial cells from the liver of patients with primary biliary cirrhosis: isolation, characterization, and short-term culture. J Pathol. 1990;162:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Joplin R, Hishida T, Tsubouchi H, Daikuhara Y, Ayres R, Neuberger JM, Strain AJ. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest. 1992;90:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Chávez R, Jamieson N, Takamori S, Nivatvongs S, Pino G, Metcalfe A, Watson C, Romero D, Metcalfe S. Hepatotrophic effect of cyclosporine and FK 506 is not mimicked by rapamycin. Transplant Proc. 1999;31:2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Sobesky R, Dusoleil A, Condat B, Bedossa P, Buffet C, Pelletier G. Azathioprine-induced destructive cholangitis. Am J Gastroenterol. 2001;96:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Horsmans Y, Rahier J, Geubel AP. Reversible cholestasis with bile duct injury following azathioprine therapy. A case report. Liver. 1991;11:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | DePinho RA, Goldberg CS, Lefkowitch JH. Azathioprine and the liver. Evidence favoring idiosyncratic, mixed cholestatic-hepatocellular injury in humans. Gastroenterology. 1984;86:162-165. [PubMed] |
| 18. | Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol. 1997;26 Suppl 1:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 101] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK