Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7541
Revised: April 23, 2005
Accepted: April 26, 2005
Published online: December 21, 2005
A 34-year-old male with hereditary non-polyposis colon cancer with a mutation in hMSH2 line is reported. Despite regular colonoscopic follow-up, he developed cecal cancer involving the extraluminal area. Due to sub-occlusive symptoms, the patient was submitted to further colonoscopy, however with no clear evidence of neoplasia. Thin slice multiplanar reconstruction computed tomography CT scan performed thereafter revealed a transmural mass 2.5 cm in size localized near the cecal valve. Discussion is made on the reliability of colonoscopic examinations as well as the need for further investigations in the follow-up of patients at very high risk of right-sided colon cancer, such as male hMSH2 carrier affected by hereditary non-polyposis colon cancer.
- Citation: Corleto VD, Zykaj E, Mercantini P, Pilozzi E, Rossi M, Carnuccio A, Giulio ED, Ziparo V, Fave GD. Is colonoscopy sufficient for colorectal cancer surveillance in all HNPCC patients? World J Gastroenterol 2005; 11(47): 7541-7544
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7541
Hereditary non-polyposis colorectal cancer (HNPCC or Lynch syndrome) is a disease of autosomal dominant inheritance, accounting for 3-5% of all colorectal cancers[1]. This disorder predisposes to the development of colorectal cancer (CRC) at an early age (mean age 45 years), as well as other cancers mainly of the gastrointestinal and genitourinary tract. HNPCC is caused by defects in one of the mismatch repair (MMR) genes such as hMLH1, hMSH2, hMSH6, hPMS1 or hPMS2, all of which are important in the detection and repair of base pair mismatches during DNA replication[2]. The presence of this syndrome is suspected on the basis of specific diagnostic guidelines known as “Amsterdam I and II, original Bethesda and revised Bethesda criteria” and on the clinical features of colorectal adenomas and/or cancers[1]. Genetic tests, to detect MMR gene mutations, are usually used to confirm the diagnosis.
Even when the more stringent clinical criteria are met, germline mutations are detected only in 40-60%[3-5]. Patients who met the family history and clinical criteria or who have inherited MMR gene mutations are at high risk of developing cancer and should undergo specific surveillance and follow-up, particularly colonic endoscopic surveillance[6,7].
Herein, we describe a case of a man with HNPCC who developed a right-sided colon cancer at the age of 34 despite regular colonoscopic examinations.
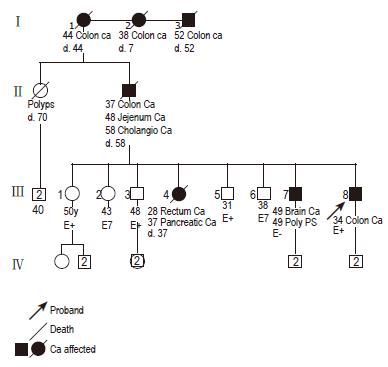
A 34-year-old man was admitted to our department with pain in the right lower quadrant of the abdomen, abdominal distension, and signs of pseudo-obstruction. The patient, with a family history of HNPCC, was regularly attended for surveillance at a well-known international center for diagnosis, treatment, and surveillance of HNPCC patients. A germline mutation in the hMSH2 gene was detected at the age of 17. A substitution at codon 383, changing CGA (Arg) to TGA (stop codon), was found which resulted in the formation of a truncated protein. No alteration was identified in the hMLH1 line. The last full endoscopic colon examination was carried out almost 2 years before and the colonic mucosa was reported with no alteration. The patient’s family pedigree is outlined in Figure 1. His father was diagnosed as having CRC at the age of 37, jejunum cancer at the age of 48 and at the age of 58 he died of cholangiocarcinoma. His older sister presented rectal carcinoma at the age of 28 and died of pancreatic cancer at the age of 37. His older brother developed colonic polyps, three times consecutively, diagnosed during colonoscopic surveillance and also presented a glioblastoma at the age of 49, although he was negative for hMSH2 mutations upon DNA analysis.
This patient had been on a semi-liquid diet for 2 d, and was treated with 20 mg of i.v. N-butylbromide for the abdominal pain. Laboratory data revealed no abnormality. Complete blood count, hemoglobin, hematocrit, ferritin, urinalysis and electrolyte panel were all within the normal range. Liver function tests, carcinoembryonic antigen (CEA) and tissue polypeptide antigen (TPA) were also normal. An abdominal X-ray showed mild colonic and ileal distension in the absence of air-fluid levels or free air below the diaphragm. Complete abdominal ultrasonic investigations revealed normal kidney, urinary tract, liver, and biliary system. A chest X-ray was also negative.
The next day colonoscopy was performed. No alteration was detected, except in the mucosa around the ileocecal valve, an irregular and protuberant appearance, above which a small sessile polyp, 7 mm in diameter was visible. Multiple biopsies were collected. Histological examination revealed only the presence of a non-specific inflammatory submucosal infiltration.
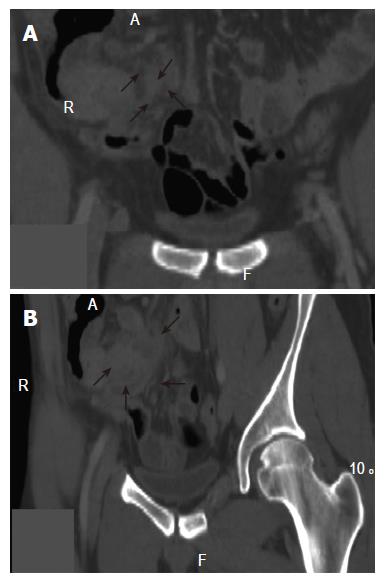
A routine spiral-CT scanning (16 slices-Mx8000 IDT, Philips, Cleveland, OH, USA) was performed and simple axial reconstructions 5 mm thick were obtained. No alterations were detected at the first examination. However, after consultation with the radiologist, a thin slice (2 mm) multiplanar reconstruction (MPR) of the right colon was performed and a 2.5-cm mass was revealed extrinsically localized to the fundus of the cecum, well visualized in the coronal planes (Figures 2A and B). Before the surgery, the patient was informed about the surgical options and the related risks and/or potential benefits. The patient agreed to undergo a right hemicolectomy, but not total colectomy. At surgery, ascites was found locally and the mass was macroscopically transmural. The surgeon, therefore, had no choice but to carry out terminal ileum resection, resection of the ileocecal valve, hepatic flexure, part of the transverse colon and dissection of related lymph nodes.
Upon histological examination, the neoplasia was found to contain moderately differentiated adenocarcinoma with transmural involvement and mucinous foci, covering 30% of the entire mass. The neoplasia was classified as T3N1MX, corresponding to Dukes’ stage C. The patient rapidly recovered after surgery.
CRC is the third most frequently diagnosed cancer and the second leading cause of cancer-related deaths in the world. The lifetime risk, in the general population, is approximately 5-6%. The cancer develops sporadically in 80% of these patients, while 20% have an inherited predisposition to the disease[8,9]. HNPCC is the most common hereditary form of CRC accounting for 3-5% of all CRCs[10,11].
HNPCC is an autosomal dominant condition that predisposes to a lifetime risk of approximately 80% of the development of CRC, localized predominantly in the proximal colon. Adenomas, in these patients, tend to have a villous component and most of them present dysplasia, compared with the more common sporadic type. The aggressive behavior of HNPCC adenomas has been demonstrated with an accelerated adenoma-carcinoma sequence (2-3 years) compared to the sporadic forms[12], which are diagnosed at a mean age of 45 and are characteristically localized in the right colon in 60-70% of these patients[13]. HNPCC mutation carriers also have a 60% lifetime risk of developing cancers in other sites of the body, predominantly the uterus, ovary, urinary tract, small bowel, and bile duct[13]. Germline mutations in hMSH2 and hMLH1 account for 90% of the mutations identified in HNPCC patients. These are scattered throughout the entire coding region without a clear phenotype-genotype correlation. However, specific sex and genotype/phenotype associations in HNPCC mutation carriers have been reported. In fact, CRC risk is reported to be higher in males than in females[14]. In one study, the CRC risk is reported to be 96% in males with a hMSH2 mutation compared with 39% in females with the same mutation[15], whereas the risk of extra-colonic cancers is reported to be 48% as compared with 11% reported in hMLH1 mutation carriers[14,15]. Based on these considerations, male hMSH2 mutation carriers should receive a tailored program of cancer surveillance. Colonoscopy is the most important tool in the surveillance for CRC; in all individuals, especially patients who are at high risk of developing a CRC, complete colonoscopy in examinations with scrupulous exploration of the cecum is always recommended[16]. Although, colonoscopy is a method of choice in colon cancer surveillance, concerns about its sensitivity and specificity in many clinical settings still remain. Complete colonoscopy refers to the passage of the colonoscope to the cecum and can be assessed by various landmarks[17]. The most reliable are the identification of the cecal valve and/or intubation of the terminal ileum[17]. The rate of colonoscopies considered to be complete decreases significantly (56.9%) when either or both of these landmarks are applied[17]. Moreover, it has been reported that only experienced endoscopists can reach the cecum in almost 97% of colonoscopies, whereas completion of the colonoscopic procedure, by self-trained endoscopists, is reported to be as low as 54%[18,19]. Various factors may cause this discrepancy, one of which might be related to the professional experience of the endoscopist who may erroneously believe that the cecum has been reached. The other factor may be related to bowel preparation that may not offer clear visualization of the cecum. The size of the cancer is also an important factor. If the neoplastic mass is very small or located in close proximity to the ileocecal valve, in an area which is difficult to explore, the cancer can be very easily missed. However, even if colonoscopy is complete, the extension of the neoplasia may be mainly extraluminal, as in the case described here.
All these factors suggest that additional diagnostic examinations, besides colonoscopy, should be made in the follow-up of very high-risk patients, such as the hMSH2 male mutation carriers, in order to complete right colon evaluation, in the event of incomplete or dubious colonoscopy. Spiral CT scan is currently used for the detection of extra-colonic cancers, which is frequently encountered in HNPCC patients. In this particularly high-risk patient, due to the specific sub-occlusive symptoms, a spiral CT scan, with thin slice multiplanar reconstruction, was used for complete visualization of the right colon, detecting a cecal cancer lesion with a predominantly extraluminal development missed at colonoscopy.
In conclusion, given the very high risk of developing a right-sided colon cancer in hMSH2 male carrier HNPCC patients and the possibility that colonoscopy may not completely visualize the cecal region, spiral CT scan should, in our opinion, be included in the follow-up of these patients. At present, due to the relative rarity of this disease, it is difficult to evaluate when, for these specific reasons, a spiral CT scan should be performed. However, besides evaluation of the clinical symptoms, surveillance of right colonic cancer with a thin slice multiplanar reconstruction spiral CT scan is advisable and should always be performed in hMSH2 male carriers in dubious cases or when colonoscopy is thought to be incomplete for any reason.
A special thanks to Mrs. Marian E. Shields for her English revision.
| 1. | Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1395] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 2. | Samowitz WS, Curtin K, Lin HH, Robertson MA, Schaffer D, Nichols M, Gruenthal K, Leppert MF, Slattery ML. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology. 2001;121:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Ponz de Leon M, Benatti P, Di Gregorio C, Pedroni M, Losi L, Genuardi M, Viel A, Fornasarig M, Lucci-Cordisco E, Anti M. Genetic testing among high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Br J Cancer. 2004;90:882-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Terdiman JP, Gum JR, Conrad PG, Miller GA, Weinberg V, Crawley SC, Levin TR, Reeves C, Schmitt A, Hepburn M. Efficient detection of hereditary nonpolyposis colorectal cancer gene carriers by screening for tumor microsatellite instability before germline genetic testing. Gastroenterology. 2001;120:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 5. | Lackner C, Hoefler G. Critical issues in the identification and management of patients with hereditary non-polyposis colorectal cancer. Eur J Gastroenterol Hepatol. 2005;17:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | American Gastroenterological Association medical position statement: hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Grady WM. Genetic testing for high-risk colon cancer patients. Gastroenterology. 2003;124:1574-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121:198-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Annie Yu HJ, Lin KM, Ota DM, Lynch HT. Hereditary nonpolyposis colorectal cancer: preventive management. Cancer Treat Rev. 2003;29:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Lynch HT, Lynch JF. Hereditary nonpolyposis colorectal cancer. Semin Surg Oncol. 2000;18:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 11. | Loukola A, Eklin K, Laiho P, Salovaara R, Kristo P, Järvinen H, Mecklin JP, Launonen V, Aaltonen LA. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res. 2001;61:4545-4549. [PubMed] |
| 12. | Lynch HT, Riley BD, Weissman SM, Coronel SM, Kinarsky Y, Lynch JF, Shaw TG, Rubinstein WS. Hereditary nonpolyposis colorectal carcinoma (HNPCC) and HNPCC-like families: Problems in diagnosis, surveillance, and management. Cancer. 2004;100:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Umar A, Risinger JI, Hawk ET, Barrett JC. Testing guidelines for hereditary non-polyposis colorectal cancer. Nat Rev Cancer. 2004;4:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW, Vogelstein B. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105-110 DOI : 10.1093/hmg/6.1.105. |
| 15. | Lin KM, Shashidharan M, Thorson AG, Ternent CA, Blatchford GJ, Christensen MA, Watson P, Lemon SJ, Franklin B, Karr B. Cumulative incidence of colorectal and extracolonic cancers in MLH1 and MSH2 mutation carriers of hereditary nonpolyposis colorectal cancer. J Gastrointest Surg. 1998;2:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Bradshaw N, Holloway S, Penman I, Dunlop MG, Porteous ME. Colonoscopy surveillance of individuals at risk of familial colorectal cancer. Gut. 2003;52:1748-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 421] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1208] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 19. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 672] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
Science Editor Ma JY and Guo SY Language Editor Elsevier HK