Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7536
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: December 21, 2005
AIM: To explore the inducing effect of human mutant p27 gene on the apoptosis of the human gastric cancer cell line MKN-45 and its associated mechanisms.
METHODS: The recombinant adenovirus Ad-p27mt was constructed to infect the human gastric cancer cell line MKN-45. Using flow cytometry, TUNEL assay and DNA fragment analysis, we measured the apoptotic effect of Ad-p27mt on the human gastric cancer cells.
RESULTS: Ad-p27mt was successfully constructed and the infection efficiency reached 100%. After 18 h of infection, we observed an apoptotic hypodiploid peak on the flow cytometer before G1-S and apoptotic characteristic bands in the DNA electrophoresis. The apoptotic rate detected by TUNEL method was significantly higher in the Ad-p27mt group (89.4±3.12%) compared to the control group (3.12±0.13%, P < 0.01).
CONCLUSION: Human mutant p27 can induce apoptosis of the human gastric cancer cells in vitro.
- Citation: Zhu JS, Wang L, Cheng GQ, Li Q, Zhu ZM, Zhu L. Apoptosis mechanisms of human gastric cancer cell line MKN-45 infected with human mutant p27. World J Gastroenterol 2005; 11(47): 7536-7540
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7536
Apoptosis plays a crucial role in the proliferation and turnover of cells in various malignant tumors. Studies have proved that apoptosis is enhanced in tumor by many anticancer drugs, such as cytotoxic drug, hormone, or some recombinant gene, medicine, etc. However, there is a lack of an effective therapy for advanced gastric cancer at present. Gastric cancer is a disease with not only abnormal cell proliferation and differentiation, but also abnormal apoptosis. So, the enhanced induction of apoptosis in human gastric cancer cell needs to be explored. Human mutant p27 gene is a kind of multifunctional cyclin-dependent kinase inhibitor, playing a negative role in cell cycle regulation by inhibiting transformation from G0/G1 phase to S phase. Similarly, a recombinant p27mt presents cell proliferation and tumorigenesis. Based on the previous studies, we examined the apoptotic effect and its associated mechanisms in human gastric cancer implanted into nude mice after the treatment with human mutant p27 gene.
BALB/C-nu/nu mice (weight 18-22 g, 36-37 wk old) and human gastric carcinoma cell line MKN-45 were obtained from Shanghai Tumor Institution (No. 01842). The animals were subcutaneously grafted with the MKN-45 cell line.
RPMI 1640 culture medium and TRIzol total RNA isolation kit were purchased from Gibco BRL. The rat anti-human p27 kip1 multi-antibody was purchased from Santa Cruz Co. The liposome, trypsin, DMEM culture medium, Hepes and Csc1, 200-bp DNA ladder, dNTP, tag enzyme and the restriction endonuclease were obtained from Sigma Co. PORF9-hp27mt plasmid (human mutant p27 gene) was obtained from InvivoGen Co. The recombinant adenovirus (Ad-lac2) and Ad-LacZ (medium of the recombinant adenovirus) were provided by Dr. Robert, Huston University, USA.
Human gastric cancer cell line MKN-45 was routinely cultured in RPMI 1640 medium supplemented with heat-inactivated 100 mL/L fetal calf serum, 100 kU/L penicillin, 100 mg/L streptomycin and 2 mmol/L glutamine, and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air until the gastric carcinoma cells were 80-90% confluent.
After pORF9-hp27mt was digested with AgeI and NheI enzymes, the 619-bp fragment was recycled and subcloned into pBluescript II SK (+), which was digested with XmaI and XbaI enzymes, resulting in pBluescript-hp27mt. pBluescript-hp27mt was digested with NotI and KpnI enzymes, and then the 699-bp fragment was recycled and inserted into shuttle plasmid vector pShuttle-CMV-hp27mt, which was digested with the same enzyme, resulting in the transfer plasmid vector pShuttle-CMV-hp27mt. The competence E. coli was transformed by adenoviral framework plasmid pAdeasy-1. According to ampicillin-resistant gene, the BJ5183 containing pAdeasy-1 was picked out and prepared as the ultra-competent BJ5183 containing pAdeasy-1. Then the ultra-competence BJ5183 containing pAdeasy-1 was transformed by transfer plasmid pShuttle-CMV-hp27mt, which was digested with PmeI enzyme and dephosphorylated by alkaline phosphatase. A little DNA of the transformed clone bacterial plasmid was taken out and the suspect DNA of the recombinant adenovirus plasmid was chosen according to the size of plasmid on agarose electrophoresis. If the chosen DNA was identified as the proper DNA by digestion with PacI enzyme, the recombinant adenovirus plasmid pAdeasy-1-hp27mt could be massively prepared, and then used to perform liposome PolyFect-mediated infection of Ad293 cells, thereby resulting in the preparation of human p27mt recombinant adenovirus (Ad-p27mt). The amplification, identification and titer determination of the recombinant adenovirus were carried out as previously described[3].
The MNK-45 cells taken from the 15-cm culture flask was infected with Ad-lacZ according to MOI of 20, 40, 50, and 100 and then incubated for another 48 h. After the cells were fixed by 0.5% glutaral pentanediol for 15 min and washed thrice with PBS, X-gal staining solution (20:1) was added, followed by incubation at 37 °C for 4-25 h in a humidified atmosphere containing 50 mL/L CO2 in air. The blue-staining cells, i.e. the positive cells in which LacZ gene was expressed, were observed under microscope and the percentage of the positive cells was calculated.
The MKN-45 cells taken from the 75-cm culture flask was infected with Ad-p27mt (MOI) and Ad-LacZ (MOI), respectively. After being incubated at 37 °C for 48 h in a humidified atmosphere containing 50 mL/L CO2 in air, all cells were digested with 0.5 g/L trypsin. The cells were collected and washed twice with PBS. After the cells were lysed with 500 μL of SDS-PAGE cell lysis solution and boiled for 5 min, they were centrifuged and the supernatant was collected for Western blot detection.
The MKN-45 cells taken from the 75-cm culture flask was infected with Ad-p27mt (MOI 100). After incubation for 48 h, they were digested with 0.5 g/L trypsin. The cells were collected and washed twice with PBS. Proper amount of PBS was added, until the cell concentration reached 109/L. Then 100 μL of cell suspension was mixed with 200 μL of DNA-PREPTM LPR, followed by detection using Coulter Epics XL flow cytometer after 15 min. The cell cycle and cell apoptosis rate were analyzed. The Ad-LacZ (MOI 100) group and the normal control group (MKN-45 cells cultivated without adenovirus) were used as control groups.
MKN-45 cells, after being infected with Ad-p27mt and Ad-LacZ for 48 h, and the normal control cells were separately collected and centrifugated at 1 000 r/min for 5 min. The supernatant was thrown away, and 500 μL of cell lysis solution [10 g/L NP40, 20 mmol/L EDTA, 50 mmol/L Tris-HCl (pH 7.5)] and 10 μL of protease K were added into the cell sediment, followed by heating in 56 °C water bath for 1-2 h and then extraction with phenol/chloroform and DNA precipitation with dehydrated alcohol. After the DNA precipitate was washed once with 700 mL/L alcohol, 200 μL of TE was added to lyse the DNA, followed by incubation with RNase (final concentration 50 mL/L) overnight at 37 °C. The final DNA was electrophoresed on 10 g/L agarose gel and observed with the aid of an ultraviolet light lamp.
Cell suspensions (1x104 cells) of each Ad-p27mt group and normal control group were inoculated separately into 60-mm dishes containing six cover glasses (washed and high-pressure sterilized), followed by incubation for 24 h. Then the glasses were taken out and washed twice with 1× PBS and fixed in methanol:freezing acetic acid (3:1) for 30 min. The following procedures were carried out according to the instructions of the kit. The average number of apoptotic cells was determined by counting 1 000 cells on each glass. Then the apoptotic index (AI), i.e. the number of apoptotic cells per 100 cancer cells, was calculated.
All statistical analyses were performed using SPSS 11.5 for Windows. The data were analyzed using t-test. A P<0.05 was considered statistically significant.
The pathological change of multi-drug resistant MKN-45 cells and their culture fluid were collected and centrifuged. Five milliliters of the supernatant was mixed with 1 mg of protease K, 2 mL of 10 g/L SDS, 10 mmol/L EDTA, and 20 mmol/L Tris-HCl to digest for 2 h. After being precipitated by dehydrated alcohol, the viral DNA was collected. PCR reaction was carried out after adding the forward and reverse primers. Finally, a 275-bp target gene was amplified, which showed that the recombinant adenovirus had already possessed p27mt target gene.
The adenovirus-mediated gene transfer rate was evaluated by X-gal staining. The results showed that the infection efficiency could reach 100% when MOI was larger than 50, which indicated that recombinant adenovirus could effectively transfer to MKN-45 cells in vitro.
The expression of p27 protein was evaluated after MKN-45 was infected with human mutant p27 recombinant adenovirus in vitro. After MKN-45 cells were infected with Ad-p27mt (MOI 100) for 24 h, the cells were collected and lysed with 1x SDS-PAGE cell lysis solution, boiled at 100 °C for 5 min, and then centrifuged. The supernatant was collected and the protein was detected by using TMB system Western blot kit (KPL, USA). We found a high expression of 27-ku protein in Ad-p27mt group, while only slight trace expression (endogenous expression) in Ad-LacZ group and normal control group, indicating that the human mutant p27 recombinant adenovirus constructed in the present study could express p27 gene properly in MKN-45 cells and the protein product could be expressed at a high level in the cells.
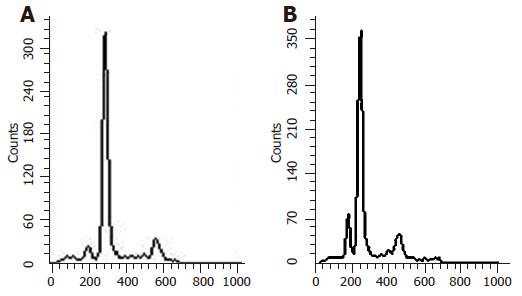
After the MKN-45 cells were treated with Ad-p27mt, Ad-LacZ and without virus for 24 h, the apoptosis rate was detected in the different groups using flow cytometry. Reproducibility was confirmed by processing all samples at least six times. The average values of hypodiploid in Ad-p27mt, Ad-LacZ, and non-infected groups were 41.0%, 4.67%, and 1.96%, respectively, showing significant differences between these three groups.
The result of DNA electrophoresis showed that the gene bands were intact in Ad-LacZ group and normal control group, while there were obvious 180-200 bp diploid ‘trapezia’ bands in Ad-p27mt group, which was in concordance with the characteristic changes of apoptosis (Figures 1 and 2).
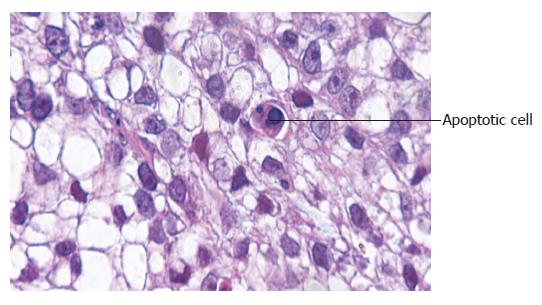
The apoptotic cells had concentrated cytoplasm with shrunk and dark-stained nuclei. AI of Ad-p27mt group (82.6±3.2%) was significantly higher compared to the control group (5.0±3.5%, P<0.05), showing that Ad-p27mt could obviously induce apoptosis of gastric cancer cells (Figure 3).
The cell cycles of different groups of MKN-45 cells are shown in Table 1. We found that the number of G0/G1 phase cells decreased gradually and the percentage of S-phase cells increased in Ad-LacZ group and control group, which indicated that the transition time of cell cycle was shortened and cell proliferation was active. However, the percentage of G0/G1 phase cells significantly increased and the cell cycle was obviously arrested in G0/G1 phase in Ad-p27mt group as compared to the control group and Ad-LacZ group (P<0.01).
Human mutant p27 protein is a kind of heat stable protein, which was first found by Polyak and colleagues in a research on cell contact inhibition with TGF-β[4]. It has been found that p27 mainly inhibited the activity of cyclin E-CDK2, cyclin D-CDK4, and cyclin A-CDK2 complexes. In addition, the arrest of cells at G0/G1 phase was mainly caused by p27 accumulation induced by exogenous signal, and tumorigenesis was closely correlated with translocation, deletion and mutation of p27 gene and changes of expression and activity of p27 protein[5]. If the expression level of p27mt was downregulated, the inhibition of cyclin E-CDK2 complex by p27 would decrease and thus the DNA damaged cells could transform from G1 to S phase directly.
The degradation of p27 protein is mainly caused by phosphorylation of the 187th threonine of p27 which is mediated by ubiquitin[6-8]. Kudo et al[9]. found that the 187th threonine of p27 was mediated by p27 protein, and thereby obviously inhibited the cell growth, and these inhibitive effects were more obvious on mutant p27 (T187A) than on wild-type p27.
In this study, we constructed a replication-deficient recombinant adenovirus, which carried p27mt, to investigate the apoptotic rate of gastric cancer cell line MKN-45, expecting for a more effective p27 gene to treat gastric cancer. Koguchi et al[11]. reported the prohibition of the viability of astrocytes, resulting from transfection with adenovirus-mediated exogenous p27 gene. Zhang et al[12]. reported that the upregulation of p27 expression by retinoic acid significantly inhibited the growth of the oophoroma cells. In addition, Koh et al[13]. found that high expression of p27 and raised expressions of cyclin D1 and cyclin E in cephalocervical squamous cell carcinoma cell lines SUN-1066, SUN-1041, and SUN-1076 after transfection with adenovirus-mediated p27kip1 could significantly inhibit the proliferation of the cancer cells by arresting the cells mainly at G1-S stage. All these data showed that p27 gene might have a significant impact on the onset, development and prognosis of tumor.
Nowadays, functional reconstruction of anti-oncogene has been a reasonable strategy of gene therapy for tumor. Sasaki et al[14]. showed that compared with p27wt, p27mt had stronger inhibitive effects on the apoptosis of cholangiocarcinoma cells. Park et al[15,16]. reported similar results when they performed adenovirus-mediated transfection of p27mt and p27wt genes into lung cancer cell lines NCI H460, NCI H1264, NCI H358, and NCI H157. In our study, adenovirus-mediated transfection of mutant p27 gene could upregulate its expression in gastric cancer cell line MKN-45, suggesting that adenovirus with reconstructed p27mt can transfect the target gene into tumor cells. We also observed that the apoptotic rate was markedly higher in Ad-p27mt group as compared to the control group. DNA analysis showed 180-200 bp DNA ladder. Moreover, TUNEL assay showed obviously higher AI up to 82.6% in Ad-p27mt group as compared to the control group. These results revealed that p27 gene might play an important role in gastric carcinoma and its downregulation may be the main cause of cell differentiation dysfunction and apoptosis dysfunction. Thus, upregulating the expression of p27 by transfection of mutant p27 may serve as a new therapy on gastric carcinoma by exerting its apoptosis-inducing effect. The cell cycle analysis showed that the cleavage of tumor cells was stopped at G1 stage via suppressing the activity of the cyclin/CDK kinase by p27mt. Winteringham et al[17]. have found that the accumulation of p27 plays a crucial role in the gastric cell cycle arrest at the initiation of cell differentiation.
In recent studies[18], although the gastric cancer cells apoptosis was successfully induced by the application of NM-3 and other gene therapies, Ad-p27mt-induced apoptosis of human gastric cancer cell line MKN-45 has not yet been reported in nude mice model. Our study showed a very useful experimental evidence for human gastric cancer suppression by using p27 gene therapy in vivo. The efficacy of p27 gene therapy and the mechanisms of apoptosis may provide an important theoretical evidence in the treatment of advanced gastric cancer.
| 1. | Nan KJ, Jing Z, Gong L. Expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27Kip1 in hepatocellular carcinoma. World J Gastroenterol. 2004;10:1425-1430. [PubMed] |
| 2. | Bryja V, Pacherník J, Faldíková L, Krejcí P, Pogue R, Nevrivá I, Dvorák P, Hampl A. The role of p27(Kip1) in maintaining the levels of D-type cyclins in vivo. Biochim Biophys Acta. 2004;1691:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Chen J, Xu SY, Deng CS, Wang JN, Huang YZ. Efficient generation of human mutant p27 gene recombinant adenovirus by homologous recombination in bacteria. J Fourth Mil Med Univ. 2004;5:406-409. |
| 4. | Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1450] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 5. | Liu E, Li X, Yan F, Zhao Q, Wu X. Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J Biol Chem. 2004;279:17283-17288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Takeda A, Osaki M, Adachi K, Honjo S, Ito H. Role of the phosphatidylinositol 3'-kinase-Akt signal pathway in the proliferation of human pancreatic ductal carcinoma cell lines. Pancreas. 2004;28:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Guo W, Shang F, Liu Q, Urim L, West-Mays J, Taylor A. Differential regulation of components of the ubiquitin-proteasome pathway during lens cell differentiation. Invest Ophthalmol Vis Sci. 2004;45:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 393] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Kudo Y, Kitajima S, Sato S, Ogawa I, Miyauchi M, Takata T. Transfection of p27(Kip1) threonine residue 187 mutant type gene, which is not influenced by ubiquitin-mediated degradation, induces cell cycle arrest in oral squamous cell carcinoma cells. Oncology. 2002;63:398-404 DOI : 10.1159/000066222. |
| 10. | Hurteau JA, Brutkiewicz SA, Wang Q, Allison BM, Goebl MG, Harrington MA. Overexpression of a stabilized mutant form of the cyclin-dependent kinase inhibitor p27(Kip1) inhibits cell growth. Gynecol Oncol. 2002;86:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Koguchi K, Nakatsuji Y, Nakayama K, Sakoda S. Modulation of astrocyte proliferation by cyclin-dependent kinase inhibitor p27(Kip1). Glia. 2002;37:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Zhang D, Holmes WF, Wu S, Soprano DR, Soprano KJ. Retinoids and ovarian cancer. J Cell Physiol. 2000;185:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Koh TY, Park SW, Park KH, Lee SG, Seol JG, Lee DW, Lee CT, Heo DS, Kim KH, Sung MW. Inhibitory effect of p27KIP1 gene transfer on head and neck squamous cell carcinoma cell lines. Head Neck. 2003;25:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Sasaki T, Katayose Y, Suzuki M, Yamamoto K, Shiraso S, Mizuma M, Unno M, Takeuchi H, Lee CT, Matsuno S. Adenovirus expressing mutant p27kip1 enhanced apoptosis against cholangiocarcinoma than adenovirus-p27kip1 wild type. Hepatogastroenterology. 2004;51:68-75. [PubMed] |
| 15. | Park KH, Seol JY, Kim TY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. An adenovirus expressing mutant p27 showed more potent antitumor effects than adenovirus-p27 wild type. Cancer Res. 2001;61:6163-6169. [PubMed] |
| 16. | Park KH, Lee J, Yoo CG, Kim YW, Han SK, Shim YS, Kim SK, Wang KC, Cho BK, Lee CT. Application of p27 gene therapy for human malignant glioma potentiated by using mutant p27. J Neurosurg. 2004;101:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Winteringham LN, Kobelke S, Williams JH, Ingley E, Klinken SP. Myeloid Leukemia Factor 1 inhibits erythropoietin-induced differentiation, cell cycle exit and p27Kip1 accumulation. Oncogene. 2004;23:5105-5109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kumagai H, Masuda T, Ohsono M, Hattori S, Naganawa H, Sawa T, Hamada M, Ishizuka M, Takeuchi T. Cytogenin, a novel antitumor substance. J Antibiot (Tokyo). 1990;43:1505-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Science Editor Kumar M Language Editor Elsevier HK