Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7520
Revised: June 1, 2005
Accepted: June 2, 2005
Published online: December 21, 2005
AIM: To investigate the effect of probiotic bacterium, Clostridium butyricum MIYAIRI 588 strain (CBM) on the changes of the fecal flora in Helicobacter pylori (H pylori) treatment.
METHODS: Thirty-five patients with gastric or duodenal ulcers positive for H pylori were randomized either to 1 wk amoxicillin, clarithromycin, lansoprazole (Group 1) or to the same regimen supplemented with CBM 7 d ahead of the triple therapy (Group 2). Stool samples were collected before and 2, 4, 7, 15, and 22 d after the starting eradication therapy, and were examined intestinal flora. Patients were required to keep a diary record of their condition.
RESULTS: Obligate anaerobes decreased significantly on d 2, 4, 8 and 15 in Group 1. On the other hand, they did not decrease significantly in Group 2. The Escherichia coli was dominant bacterium in Enterobacteriaceae, but that was replaced by other species such as Klebsiella and Enterobacter after eradication in Group 1. The change was suppressed in Group 2. Abdominal symptoms were less frequent in Group 2 than in Group 1.
CONCLUSION: The combined use of CBM reduced the changes in the intestinal flora and decreased the incidence of gastrointestinal side effects.
- Citation: Shimbo I, Yamaguchi T, Odaka T, Nakajima K, Koide A, Koyama H, Saisho H. Effect of Clostridium butyricum on fecal flora in Helicobacter pylori eradication therapy. World J Gastroenterol 2005; 11(47): 7520-7524
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7520.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7520
Antibiotic therapy causes loose stools and/or diarrhea quite frequently, probably because of changes in the intestinal flora. Super infection arising from the alterations of the normal intestinal flora is a factor in the onset of infectious enteritis.
Helicobacter pylori (H pylori) is deeply involved in gastroduodenal ulcer disease and H pylori -positive patients are generally treated with antibiotics to eradicate this bacterium[1]. Triple therapy with a proton pump inhibitor and two antibiotics selected from among the following three: amoxicillin, clarithromycin and metronidazole, is now considered to be the standard therapy, and it is reported that a bacterial eradication rate of about 80-90% can be achieved[2-4]. Although such therapy causes few serious adverse reactions, gastrointestinal side effects like diarrhea and loose stools occur at a high incidence[5].
It is known that probiotic supplementation prevents or reduces of antibiotics-induced diarrhea. Recent studies have shown that probiotics were effective against gastrointestinal symptoms associated with H pylori eradication therapy[6]. Probiotics are defined as viable microorganisms which, when ingested, have a beneficial effect on human health, including amelioration or prevention of specific pathologic conditions[7].
Clostridium butyricum is a butyric acid producing Gram-positive anaerobe which is found in soil and the intestines of healthy animals and humans, and the MIYAIRI 588 strain of C. butyricum (CBM) has been used as probiotics for treating and preventing non-antimicrobial induced diarrhea, antimicrobial associated diarrhea and constipation in human beings[8-10]. MIYA-BM® tablets (Miyarisan pharmaceutical Co., Ltd., Tokyo, Japan) containing CBM were approved from Japanese Ministry of Health and Welfare for human clinical use since 1970 in Japan.
However, the preventive effect of CBM preparation on the abnormalities of intestinal microflora due to H pylori eradication therapy was unknown. Therefore, in the present study, we have examined the changes of fecal flora and gastrointestinal symptoms frequently during H pylori eradication therapy, and evaluated the utility of CBM supplementation to prevent the disturbance of the intestinal flora.
Of the patients diagnosed as having gastric or duodenal ulcers at the outpatient clinic of the First Department of Medicine of Chiba University, 35 patients (male/female: 28/7; mean age 52.7±11.3 years) were enrolled in this study. They were all positive for H pylori by culture method, microscopical examination and the rapid urease test (PyloriTek® test kit, Serim Research Corp., Elkhart, IN, USA).
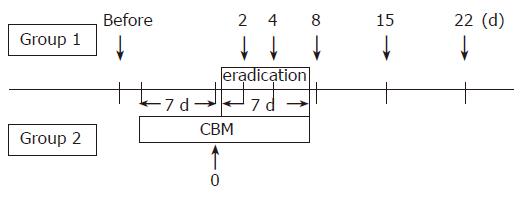
The patients were blindly and randomly allocated to two groups. Seventeen patients were assigned the 1 wk triple therapy consisting of amoxicillin 1 500 mg b.i.d., clarithromycin 400 mg b.i.d. and lansoprazole 60 mg b.i.d. (Group 1). Alternatively, eighteen were assigned to the same regimen supplemented with CBM (120 mg t.i.d.), which was started 1 wk prior to the eradication therapy for 14 d (Group 2). The patients had not been treated with any drugs that might have influenced intestinal bacteria, and had no known underlying gastrointestinal disorders. They were all given a full explanation of the contents of the study and they provided their informed consent in writing.
Six weeks after the completion of the therapy, the 13C-urea breath test was performed to check whether eradication had been achieved.
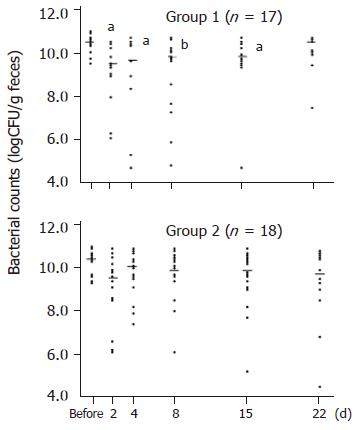
Stool samples were collected the day before the start of the therapy, and on d 2, 4, 8, 15, and 22 after the start of H pylori eradication therapy in Group 1. In Group 2, stools were also collected 1 wk after the administration of CBM (Figure 1). Fresh samples were placed in a transporter filled with CO2 (Kenkiporter® II, Clinical Supply, Gifu, Japan), and were transferred under refrigeration for culture within 24 h of the collection.
Intestinal flora was investigated according to Mitsuoka’s method[8]. Any bacterium detected was identified at the level of species or genus, and counted. The microorganisms were classified into sub groups of bacteria according to morphology of the colonies, Gram staining and cell shapes. Isolation of Clostridium difficile was also attempted.
The nonselective media used for isolation included TS, EG, and BL agar, while selective media included DHL, TATAC, P, PEES, LBS, NBGT, ES, BS, and CCMA agar. A stool sample (0.5 g) was placed into an anaerobic glove box (N2 80%, H2 10%, CO2 10%) and was diluted with a diluent for anaerobic bacteria from 10-fold to 108-fold in 10 steps. Aliquots (0.05 mL) of each dilution were cultured anaerobically in an incubator in the glove box at 37 °C for 3 d. When TS, DHL, TATAC, P, or PEES agar was used the culture plates were removed from the glove box after the bacteria were seeded and were cultured aerobically at 37 °C for 1 to 2 d. Any colony that grew during anaerobic culture was also examined for aerobic growth. Smears were prepared on slide glasses for any colony that grew and the smears were subjected to Gram staining for microscopic observation. Then each organism was classified on the basis of its characteristics, including colony morphology, growth under aerobic conditions, and growth in selective culture media. The number of live bacteria of each genus per 1 g of feces was calculated from the number of colonies and converted into a logarithmic equivalent for each bacterial species identified; log colony forming unit/g feces (logCFU/g feces). The detection limit was 2.3 logCFU/g feces. The total count of viable bacteria was calculated from the sum of the counts of each bacterial species. Species in the Enterobacteriaceae were identified by the kit of Enterotube® II (Becton Dickinson, USA).
Each patient was required to keep a daily record of the conditions of their stools and any abdominal symptoms, from before the therapy to 2 wk after the completion of H pylori eradication therapy.
Bacterial counts were expressed as the mean±SD. Statistical evaluation of changes within groups was carried out using the Wilcoxon signed-rank test. The Mann-Whitney U test was used for comparison between groups. Fisher’s exact test was performed to compare the detection rates. Differences of P<0.05 were considered to be statistically significant.
The levels of obligate and facultative anaerobes in the groups did not differ significantly. The predominant bacteria were obligate anaerobes, such as Bacteroidaceae, Bifidobacteria and in both groups (Table 1). As for the viable counts and detection rates of all species, there was not significant difference between the two groups. The viable counts and detection rates of all species did not change significantly between before and 1 wk after CBM administration in Group 2 (data not shown). None of the patients was positive for C. difficile as assessed by culture.
| Population | LogCFU/g | |||
| Group 1 (n = 17) | Group 2 (n = 18) | |||
| Total counts | 10.4±0.4 | 10.2±0.5 | ||
| Obligate anaerobes | 10.4±0.4 | 102±0.6 | ||
| Bacteroidaceae | 10.1±0.4 | (100) | 9.3±1.6 | (100) |
| Bifidobacteria | 9.8±0.6 | (93.8) | 9.6±0.6 | (93.8) |
| Peptococci | 8.9±0.8 | (62.5) | 9.0±0.5 | (62.5) |
| Clostridia | 7.5±1.9 | (93.8) | 7.9±1.5 | (100) |
| Micrococci | 3.0±1.2 | (25) | 3.5±1.8 | (37.5) |
| Facultative anaerobes | 8.3±0.7 | 8.1±1.0 | ||
| Enterobacteriaceae | 7.2±1.7 | (93.8) | 6.7±1.5 | (100) |
| E.coli | 7.3±1.4 | (81.3) | 6.4±1.6 | (93.8) |
| Others | 6.9±1.8 | (18.8) | 5.7±1.0 | (31.3) |
| Enterococci | 7.3±1.1 | (93.8) | 7.9±1.0 | (93.8) |
| Lactobacilli | 7.2±1.6 | (100) | 6.9±1.0 | (93.8) |
| Bacilli | 7.6±0.9 | (37.5) | 7.4±0.6 | (25) |
| Yeasts | 5.0±2.0 | (75) | 4.8±1.8 | (56.3) |
Counts of CBM increased and averaged 6.7 logCFU/g on the day before eradication therapy. The detection rate was the highest on d 2 (90%); it gradually decreased. On d 15, CBM became under detectable.
Obligate anaerobes decreased significantly on d 2, 4, 8, and 15, and returned to their pretreatment levels on d 22 in Group 1. On the other hand, the number of obligate anaerobes did not change significantly at any time in Group 2 (Figure 2). The number of facultative anaerobes did not decrease significantly in either group.
The detection rates of intestinal flora are shown in Table 2. Bacteroidaceae, the dominant bacteria, did not change after starting eradication of H pylori in either group. Bifidobacterium was significantly lower on d 2, 4, and 8 in Group 1, in the other, only on d 4 in Group 2. As for each group of facultative anaerobes, E.coli was significantly lower on d 4, 8 and other species such as Klebsiella and Enterobacter in Enterobacteriaceae were significantly higher on d 2, 4, 8, and 15 in Group 1. On the other hand, either Enterobacteriaceae did not change significantly in Group 2. Lactobacilli significantly lower in either group.
| Detection rates (%) | |||||||
| Population | Group | Before | D2 | D4 | D8 | D15 | D22 |
| Bacteroidaceae | 1 | 100 | 100 | 93.8 | 100 | 100 | 93.3 |
| 2 | 100 | 94.4 | 100 | 100 | 100 | 94.4 | |
| Bifidobacteria | 1 | 93.8 | 62.5a | 50.0b | 62.5a | 68.8 | 73.3 |
| 2 | 93.8 | 66.7 | 44.4b | 72.2 | 66.7 | 66.7 | |
| Clostridia | 1 | 93.8 | 93.8 | 75 | 100 | 100 | 100 |
| 2 | 100 | 94.4 | 94.4 | 94.4 | 100 | 100 | |
| Enterobacteriaceae | 1 | 93.8 | 93.8 | 100 | 93.8 | 100 | 100 |
| 2 | 100 | 94.4 | 100 | 94.4 | 100 | 100 | |
| E.coli | 1 | 81.3 | 62.5 | 25 | 37.5a | 87.5 | 93.3 |
| 2 | 93.8 | 72.2 | 94.4 | 66.7b | 88.9 | 77.8 | |
| Others | 1 | 18.8 | 75.0b | 81.3d | 75 | 81.3d | 46.7 |
| 2 | 31.3 | 27.8 | 16.7 | 38.9 | 33.3 | 50 | |
| Enterococci | 1 | 93.8 | 87.5 | 81.3 | 100 | 100 | 100 |
| 2 | 93.8 | 100 | 100 | 100 | 100 | 100 | |
| Lactobacilli | 1 | 100 | 87.5 | 68.8a | 43.8d | 93.8 | 93.3 |
| 2 | 93.8 | 61.1a | 61.1a | 44.4b | 100 | 88.9 | |
| Yeasts | 1 | 75 | 93.8 | 100.0a | 93.8 | 87.5 | 86.7 |
| 2 | 56.3 | 61.1 | 72.2 | 83.3 | 44.4 | 66.7 | |
Yeasts was significantly higher on d 4 in Group 1. No significant alterations in Yeasts were observed in Group 2. All bacterial groups recovered to pretreatment levels d 22 after completion of the drug administration. No patients were colonized with C.difficile at any time.
As for abdominal symptoms, in Group 1, loose stools were noted in 8 of 17 patients (47.1%): diarrhea in 2 of 17 patients (11.8%): abdominal pain and distention in 2 of 17 patients (11.8%), each. In Group 2, loose stools were noted in 4 of 18 patients (22.2%) and diarrhea in 1 of 17 patients (5.6%). The rates were lower in Group 2, although the differences were not significant (Table 3). Treatment was successful in 13 out of 17 patients in Group 1 (76.5%) and in 17 out of 18 patients in Group 2 (94.4%).
| Group 1 (n = 17, %) | Group 2 (n = 18, %) | |||
| Loose stool | 8 | (47.1) | 4 | (22.2) |
| Water diarrhea | 2 | (11.8) | 1 | (5.6) |
| Abdominal pain | 2 | (11.8) | 0 | (0) |
| Abdominal distention | 2 | (11.8) | 0 | (0) |
The intestinal flora in our subjects before the treatment was similar to that detected in normal adults by Mitsuoka[11]. It is known that the normal fecal flora is almost the same as that of the recto-sigmoid colon. Intestinal flora is reported to remain stable in each individual, although it differs among individuals[12,13]. However, it has been shown that the administration of antimicrobial agents, which are excreted in the bile, in the intestinal mucosa or are incompletely absorbed, causes changes in the normal intestinal flora.
Buhling et al[14]. have shown that infrequent detection of Clostridia, Veillonela, Eubacteria, Actinomyces, Bifidobacteria and E.coli with simultaneous more frequent growth of other Enterobacteria and Yeasts on d 8 of starting H pylori treatment, and after 4 wk of therapy, the microflora returned to normal.
When they collected stool samples for three times (d 0, 8, 35-40), we examined the chronological changes of the intestinal flora were followed 6 or 7 times at short intervals during and after H pylori treatment in this study.
Then we found that the total bacterial counts started to decrease as early as on d 2 of therapy. Especially, obligate anaerobes, which are the dominant bacteria, were markedly reduced. And it took 3 wk for them to return to their pretreatment levels after starting of therapy.
These bacteria are known to produce short-chain fatty acids (SCFA). Such SCFA are considered to promote proliferation of colonic epithelial cells, provide energy for colonic epithelial cells and to stimulate the absorption of water and sodium[15-19].
Previous studies have demonstrated a close correlation between a decrease in the viable count of anaerobes and a reduction in SCFA content. The drastic reduction of intestinal SCFA associated with the decrease in anaerobes during the diarrheal stage and the increase of the pH thought to be due to these changes, result in increase of the fecal water content[20-22].
Probiotics are known to prevent or lower the antibiotic-related gastrointestinal side effects. It is reported that CBM together with germinated barley foodstuff effectively increased the level of SCFA in feces and suppressed dextran sulfate sodium-induced experimental colitis in rats[23]. Butyric acids, which is produced by CBM has stronger stimulatory effects on epithelial cell proliferation than other SCFA, such as acetic acid or propionic acid[24].
Tanaka et al[25]. reported that the effect of CBM on the side effect of H pylori eradication triple therapy in vivo. These results have shown that obligate anaerobe and Lactobacillus decreased in the rat intestine and then SCFA in intestinal contents were decreased. However, administration of CBM preparation with H pylori eradication triple therapy was increased SCFA and resident fusiform bacteria in the mucous layer were more frequently observed in rats administered with CBM preparation than H pylori eradication triple therapy.
Our present findings show that CBM preparations prevent the decrease of obligate anaerobes and reduce the frequency of gastrointestinal side effects. These result similar to the pervious in vivo result. However, we did not examine the concentration of SCFA in this study but it is possible that the one of mechanism of action of CBM preparation to prevent the side effect induced by not only maintenance of intestinal flora but also increased SCFA contents by CBM.
In our study, H pylori eradication therapy caused a significantly greater decrease of E.coli , as a results, Enterobacteriaceae, except of E.coli, such as Klebsiella and Enterobacter that are known to potential cause of diarrhea was rising in Group 1.
Our results showed that CBM suppressed the replacement of E.coli by other species in Entero-bacteriaceae and superinfection.
In conclusion, intestinal flora with an eradication treatment of H pylori not a little changed. The change was reduced by supplementation of CBM, and the frequency of gastrointestinal side effects decreased. Furthermore CBM may have some beneficial effects on H pylori infection.
We would like to express our gratitude to Mamoru Tanaka, Miyarisan pharmaceutical Co., Ltd. for his technical help.
| 1. | Bakir AA, Levy PS, Dunea G. The prognosis of lupus nephritis in African-Americans: a retrospective analysis. Am J Kidney Dis. 1994;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Miwa H, Nagahara A, Sato K, Ohkura R, Murai T, Shimizu H, Watanabe S, Sato N. Efficacy of 1 week omeprazole or lansoprazole-amoxycillin-clarithromycin therapy for Helicobacter pylori infection in the Japanese population. J Gastroenterol Hepatol. 1999;14:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Labenz J, Stolte M, Peitz U, Tillenburg B, Becker T, Börsch G. One-week triple therapy with omeprazole, amoxycillin and either clarithromycin or metronidazole for cure of Helicobacter pylori infection. Aliment Pharmacol Ther. 1996;10:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Wurzer H, Rodrigo L, Stamler D, Archambault A, Rokkas T, Skandalis N, Fedorak R, Bazzoli F, Hentschel E, Mora P. Short-course therapy with amoxycillin-clarithromycin triple therapy for 10 days (ACT-10) eradicates Helicobacter pylori and heals duodenal ulcer. ACT-10 Study Group. Aliment Pharmacol Ther. 1997;11:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdorffer E, O'Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1:138-144 DOI : 10.1111/j.1523-5378.1996.tb00027.x. |
| 6. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Gorbach SL. Lactic acid bacteria and human health. Ann Med. 1990;22:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 101] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Seki H, Shiohara M, Matsumura T, Miyagawa N, Tanaka M, Komiyama A, Kurata S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr Int. 2003;45:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Kamiya S, Taguchi H, Yamaguchi H, Osaki T, Takahashi M, Nakamura S. Bacterioprophylaxis using Clostridium butyricum for lethal caecitis by Clostridium difficile. Rev. Med. Miclobiol. 1997;8:S57-S59. |
| 10. | Okamoto T, Sasaki M, Tsujikawa T, Fujiyama Y, Bamba T, Kusunoki M. Preventive efficacy of butyrate enemas and oral administration of Clostridium butyricum M588 in dextran sodium sulfate-induced colitis in rats. J Gastroenterol. 2000;35:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Mitsuoka T. Cultivation of intestinal bacteria. In: Mitsuoka T, ed. Intestinal bacteria and health. Tokyo: Harcourt Brace Jovanovich Japan 1978; 19-36. |
| 12. | Sanders WE, Sanders CC. Modification of Normal Fora by Antibiotics: Effects on Individuals and the Environment. New dimensions in antimicrobial therapy. In: Root RK, Sande MA, eds. New York: Churchill Livingstone 1984; 217-41. |
| 13. | Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci. 1986;31:147S-162S. [PubMed] |
| 14. | Bühling A, Radun D, Müller WA, Malfertheiner P. Influence of anti-Helicobacter triple-therapy with metronidazole, omeprazole and clarithromycin on intestinal microflora. Aliment Pharmacol Ther. 2001;15:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 15. | Sakata T, von Engelhardt W. Stimulatory effect of short chain fatty acids on the epithelial cell proliferation in rat large intestine. Comp Biochem Physiol A Comp Physiol. 1983;74:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424-429. [PubMed] |
| 17. | Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG. Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500-1507. [PubMed] |
| 18. | Koruda MJ, Rolandelli RH, Settle RG, Saul SH, Rombeau JL. Harry M. Vars award. The effect of a pectin-supplemented elemental diet on intestinal adaptation to massive small bowel resection. JPEN J Parenter Enteral Nutr. 1986;10:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Crump MH, Argenzio RA, Whipp SC. Effects of acetate on absorption of solute and water from the pig colon. Am J Vet Res. 1980;41:1565-1568. [PubMed] |
| 20. | Tazume S, Ozawa A, Yamamoto T, Takahashi Y, Takeshi K, Saidi SM, Ichoroh CG, Waiyaki PG. Ecological study on the intestinal bacteria flora of patients with diarrhea. Clin Infect Dis. 1993;16 Suppl 2:S77-S82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Fujita K, Kaku M, Yanagase Y, Ezaki T, Furuse K, Ozawa A, Saidi SM, Sang WK, Waiyaki PG. Physicochemical characteristics and flora of diarrhoeal and recovery faeces in children with acute gastro-enteritis in Kenya. Ann Trop Paediatr. 1990;10:339-345. [PubMed] |
| 22. | Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157: H7 infection in mice. FEMS Immunol Med Microbiol. 2004;41:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Araki Y, Fujiyama Y, Andoh A, Koyama S, Kanauchi O, Bamba T. The dietary combination of germinated barley foodstuff plus Clostridium butyricum suppresses the dextran sulfate sodium-induced experimental colitis in rats. Scand J Gastroenterol. 2000;35:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Kripke SA, Fox AD, Berman JM, Settle RG, Rombeau JL. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr. 1989;13:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 220] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Tanaka M, Miyagawa N, Tsutsumi Y. Preventive effect of Clostridium butyricum on the side effect of the Helicobacter pylori eradication. Chin J Gastroenterol. 2000;5:Suppl A116. |
Co-first-authors: Izumi Shimbo, and Taketo Yamaguchi
Co-correspondents: Taketo Yamaguchi
Science Editor Guo SY Language Editor Elsevier HK