Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7480
Revised: April 2, 2005
Accepted: April 8, 2005
Published online: December 21, 2005
AIM: To analyze the relationship between perisinusoidal stellate cell (PSC) activation and the dietary fat quantity and composition in the treatment of hepatic steatosis.
METHODS: Using an experimental rat model of steatosis based on the intake of a hyperlipidic diet (14% fat as olive oil or sunflower oil, HL-O and HL-S, respectively), we analyzed the liver’s capability of recovery after the treatment with a normal-lipidic diet (5% fat as olive oil or sunflower oil, NL-O and NL-S, respectively) by immunocytochemical and Western blot analysis of glial fibrillary acidic protein (GFAP) expression in PSCs, collagen quantification and serum aminotransferase determination.
RESULTS: The fatty infiltration in the steatotic livers decreased after the treatment with both NL diets, indicating liver recovery. This decrease was accompanied with a lower collagen deposition and aminotransferase level as well as changes in the PSC population that increased the GFAP expression. The above-mentioned effects were more pronounced in animals fed on NL-O based diet.
CONCLUSION: Treatment with a balanced diet enriched in olive oil contributes to the liver recovery from a steatotic process. The PSC phenotype is a marker of this hepatic-recovery model.
- Citation: Hernández R, Martínez-Lara E, Cañuelo A, Moral MLD, Blanco S, Siles E, Jiménez A, Pedrosa J&, Peinado M. Steatosis recovery after treatment with a balanced sunflower or olive oil-based diet: Involvement of perisinusoidal stellate cells. World J Gastroenterol 2005; 11(47): 7480-7485
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7480.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7480
Hepatic steatosis is associated with obesity[1,2], toxic injury by toxins such as alcohol[3] or thioacetamide[4], and intake of high quantities of dietary fat[1,4]. Particularly, high-fat diets lead to biochemical and morphological hepatic changes, including steatosis and collagen deposition as well as alterations in the levels of plasma lipoproteins and serum aminotransferases (ALT, AST)[1,4,5]. These features are affected not only by the quantity but also by the composition of the dietary fat [1,5,6].
Perisinusoidal stellate cells (PSCs), also known as Ito cells or fat-storing cells, are liver pericytes that are involved in the hepatic metabolism of lipoproteins[7], synthesis of extracellular matrix proteins[8] and release of some hepatocyte growth factors[9], thus playing a key role in different pathologies such as hepatic fibrosis[10] and non-alcoholic steatohepatitis[11]. In addition, these cells due to their privileged location among hepatocytes, endothelial cells and nerve endings, have been proposed to be the main cell type responsible for the microcirculation control at the sinusoidal level[12].
In the normal liver, PSCs show a quiescent phenotype characterized by the presence of numerous intracytoplasmic fat droplets[13]. Nevertheless, in different hepatic pathologies, these cells are detected without lipid droplets showing an activated phenotype that implies changes in their antigenic composition[14,15] as well as in their morphology and functions[15,16].
PSCs share several features with astrocytes[16,19] and contain the glial fibrillary acidic protein (GFAP), a type of intermediate filament protein like these glial cells[20]. The plasticity in the expression of this cytoskeletal protein in PSCs has been proposed to be an instrument to discriminate between quiescent and activated phenotypes[20].
The aim of the present study was to analyze the relationship between PSC activation and dietary fat quantity and composition in the treatment of hepatic steatosis. For this, an evaluation of the expression and distribution of the liver glial fibrillary acidic protein was made, and the liver collagen and the aminotransferase serum level were quantified in a rat model. These diets were prepared with olive or sunflower oils to compare the efficacy of the two fats on steatosis recovery.
Semipurified and balanced high-lipidic (HL; 14% fat) and normal-lipidic (NL; 5% fat) sunflower oil and olive oil diets were prepared as previously described[1,4]. The only difference between HL and NL diets was that the fat energy content was reduced from 14% to 5%, with a corresponding increase in the carbohydrate energy content. To avoid auto-oxidation, the oils were added to the diets on a daily basis immediately before feeding. All the animals had free access to the diet and water.
A total of 32 weaned Wistar rats were divided into two groups of 16 rats each and fed ad libitum on high-lipidic olive (HL-O) or high-lipidic sunflower (HL-S) oil diets for 1 mo. This time period was long enough to induce steatotic pathology[1,4]. Half of the rats of each HL oil group (n = 8 olive, n = 8 sunflower) were killed, and the other half were fed on a normal-lipidic (NL-O, NL-S) diet without changing the type of oil for an additional month.
The experiments followed the European Union guidelines on the use of animals for biomedical research (86/609/EU).
Four rats from each group were intraperitoneally anaesthetized with Ketolar (15 mg/100 mg body weight), and heparin was injected through the penis dorsal vein (500 IU/kg body weight) to avoid blood coagulation. The livers were perfused with 20-30 mL of carbogenated 0.01 mol/L phosphate buffer saline (PBS), and then with 100-120 mL of 4% paraformaldehyde-0.1 mol/L phosphate buffer (PB). After this, the organ was dissected out, cut into small blocks and immersed in the same fixative for 3 h at 4 ºC, and then immersed in 30% sucrose-0.1 mol/L phosphate buffer overnight at 4 ºC. The blocks were covered with OCT compound and frozen with 2-methylbutane at liquid-nitrogen temperature.
Histological study for the analysis of fatty infiltration was performed on 20-μm-thick sections stained with 1% OsO4 0.1 mol/L phosphate buffer for 1 h at 4 ºC and mounted in PBS:glycerol (1:1).
Free-floating sections (40-μm thick) were obtained for collagen quantification by the colorimetric method described elsewhere[21].
For immunohistochemical analysis, free-floating (40-μm thick) sections were incubated overnight with a rabbit polyclonal anti-bovine GFAP (1:100; DAKO A/S, Z334) antiserum diluted in PBS containing 0.2% Triton X-100 at 4 °C. After several washes in PBS, the sections were incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories Ltd) followed by peroxidase-linked ABC. The peroxidase activity was demonstrated following the nickel-enhanced diamino-benzidine procedure[24]. The sections were then mounted on slides, dehydrated in ascending grades of ethanol series, and covered using DPX. Controls for background staining, which was usually negligible, were performed by replacing the primary antiserum with normal rabbit serum.
Four rats from each group were killed by cervical dislocation and the livers were individually triturated with liquid nitrogen. The resulting powder was kept at -80 ºC and used for the preparation of crude extracts. Frozen liver powder (0.3 g) was homogenized (Polytron, PT 1200) in 30 mmol/L Tris-HCl buffer, pH 7.4, containing 0.5 mmol/L DTT, 1% SDS, 1 mmol/L EDTA and 1 mmol/L PMSF (1:3 w/v). The resulting homogenates were centrifuged for 60 min at 100 000 g. All the procedures were performed at 0-4 ºC. Protein concen-trations in the supernatants were determined by the Bradford method[23].
For Western blot analysis, equal amount of the denatured proteins per lane (30 μg) were loaded and separated on an 8% SDS-polyacrylamide gel (Mini Protean II, BioRad), and transferred to a PVDF membrane (Hybond-P, Amersham). The membrane was blocked with 5% defatted milk powder in Tris-buffered saline buffer (25 mmol/L Tris-HCl, pH 7.6, 137 mmol/L NaCl, 2.6 mmol/L KCl, 0.2% Tween-20) and incubated at room temperature with diluted rabbit polyclonal anti-cow GFAP antibody (1/1 000) in a blocking buffer. Bound antibodies were revealed with an enhanced chemiluminescence kit (ECL, Amersham) according to the manufacturer's instructions. After immunodetection, membranes were probed with anti α-tubulin (Sigma) as a loading control. The relative expression of GFAP in each sample was quantified by densitometric scanning.
Blood samples were taken from the mesenteric vein of the same animals used for Western blot analysis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were automatically analyzed with a multifunctional biochemistry analyzer (Autoanalyzer Hitachi 917, Roche).
Data were expressed as mean±SD. Student’s t-test was performed to evaluate significant differences between groups. P<0.05 was considered statistically significant.
Table 1 lists the body weight and liver weight before and after hyperlipidic and normal-lipidic diets.
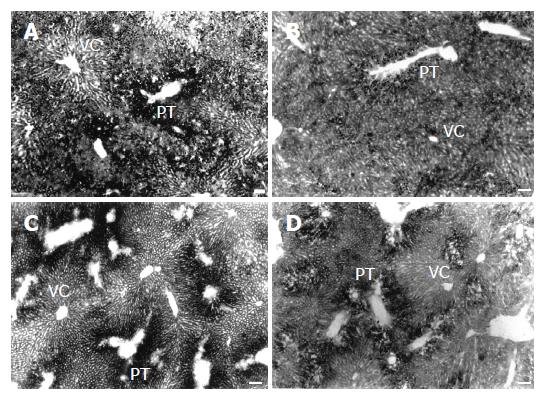
Sections stained with OsO4 revealed steatosis in the HL groups[4], characterized by the accumulation of many fat droplets inside the hepatocytes, mainly in those from the portal zone. After treatment with NL diets, steatosis was reduced principally in the NL-O group (Figure 1).
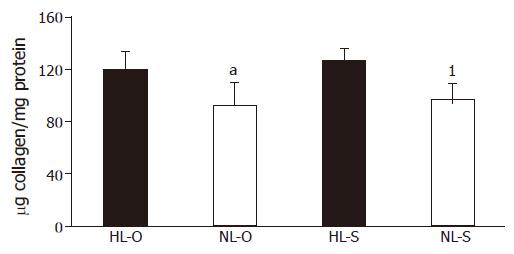
Regardless of the type of diet, the collagen quan-tification significantly decreased in rats after treatment with NL diets (Figure 2).
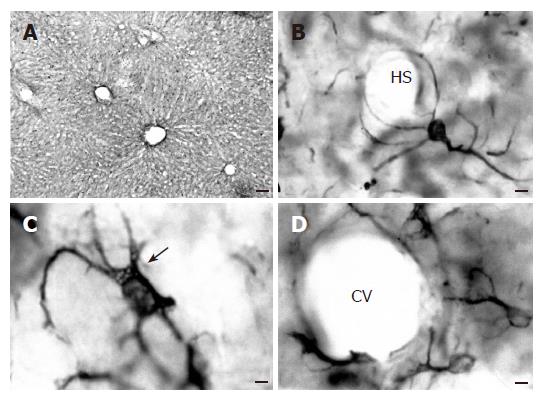
In all experimental groups, glial fibrillary acidic protein immunoreactive (GFAP-IR) cells were uniformly distributed in the liver parenchyma throughout the hepatic lobule (Figure 3A). These cells had a stellate shape with a large nucleus surrounded by a cytoplasm that exhibited many long processes (Figures 3B-D). Most of the cells contained numerous lipid droplets in their cytoplasm (Figure 3C), though some cells lacked fat droplets (Figure 3B).
The GFAP-IR cells had their cytoplasmic processes preferentially circumscribed to the Disse’s space, running along or encircling the hepatic sinusoids (Figure 3B). Nevertheless, some cells, probably those called the "second layer cells"[24] located near the vascular network of the liver, sent out some processes towards the wall vessel, where they formed structures resembling the end-feed of astrocytes (Figures 3B and 3D).
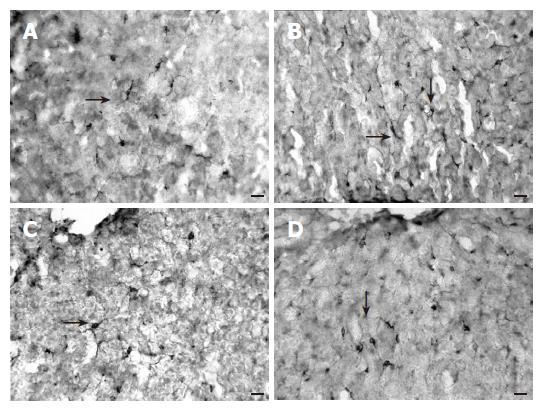
When different dietary groups were compared, a lower frequency of GFAP-IR cells was found in the steatotic liver of animals fed on HL diets (sunflower or olive oil) in relation to the group receiving NL balanced diets (sunflower or olive oil) (Figure 4). No differences were found when diets with the same lipid content but with a different oil were compared.
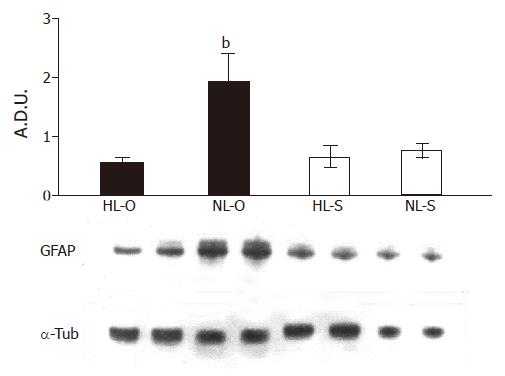
Western blot analysis of denatured hepatic homogenates from HL and NL groups revealed a 51-ku protein detected by the GFAP antibody (Figure 5). The densitometric semiquantification showed that, after the normalization of the fat level in the diets (5%), GFAP expression was significantly increased but only in the olive oil diet (P<0.001).
Serum ALT and AST levels are shown in Table 2. Compared with the HL groups, serum ALT activity significantly decreased after the treatment with NL diets. This decrease was more significant in the sunflower oil group (P<0.01). AST activity decreased only in the olive oil group after the treatment with NL diet (P<0.05).
Several studies have emphasized the importance of dietary composition in the treatment of fatty liver[5,24]. Our results based on the induction of a steatotic process by diets of 14% fat and its recovery by diets of 5% fat demonstrated that these NL diets could reduce the collagen deposition and aminotransferase serum level as well as changes in the PSC population that increased the GFAP expression. In addition, taking into account that both HL and NL diets were prepared with sunflower or olive oils, we found that the above-mentioned effects were more pronounced in animals fed on NL olive oil-based diet.
No information is available on the involvement of PSCs in the regenerative potential of steatotic liver induced by high-lipidic diets, even though it is well known in other models of liver injuries. GFAP is the most reliable marker for testing the functional status of these liver cells[20]. In this sense, we used the immunohistochemical study of GFAP as well as its expression by Western blot to evaluate the dynamics of the PSC changes. Our results showed a significant increase in the GFAP expression after the treatment of steatotic animals with a NL-O diet.
The GFAP is an essential molecule for the formation and maintenance of the characteristic cytoplasmic processes[12]. Moreover, the role of PSCs in lipid storage and metabolism[7,17], synthesis of growth factors, and particularly in regulation of the hepatic microcirculation[10] is crucial in the development of and recovery from steatosis. In fact, the location of GFAP-IR cells surrounding the hepatic sinusoids, and also forming a peculiar barrier with their processes ending in the vascular wall of the periportal and pericentral vessels, allows us to suggest the participation of PSCs not only in the lipoprotein metabolism but also in the lipoprotein exchange between the Disse’s space and the blood stream. Indeed, it has been reported that during hepatocyte proliferation after partial hepatectomy, PSCs send out their processes between hepatocytes and in vascular spaces, allowing the restoration of hepatic vascularization[25]. Thus, the present results showing GFAP increases in the PSCs population after the treatment with NL-O may be correlated with the recovery from the steatotic process.
It was reported that the type of fat in the diet affects serum lipid levels[1,4], as well as the quantity and spatial distribution pattern of the fat stored in hepatocytes[4]. It is noteworthy that, under our experimental conditions, both the doses and the type of dietary fat effectively influenced GFAP expression and the activation degree of PSCs. It has been shown that activation of PSCs by peroxidation reactions is induced by free radicals[26,27]. In this sense, polyunsaturated fatty acids (PUFAs) from sunflower oils can be more easily affected by oxidative stress. Consequently, given that NL-S diet does not significantly augment GFAP expression in the liver, we might conclude that PSCs from animals fed on the sunflower-oil diet are more strongly activated than those from animals fed with olive oil containing mainly monounsaturated fatty acids (MUFAs), which are less sensitive to peroxidation.
Hepatic steatosis due to excessive dietary fat contents is characterized by an increased collagen deposition, like other hepatic pathologies such as cirrhosis and fibrosis[28,29]. Previous studies carried out in fibrotic rats showed that olive oil, in contrast to polyunsaturated oils, could protect against the development of fibrosis[30] though this depends on the experimentally induced fibrotic model that was employed[4]. It is therefore interesting to determine how the quality of fat in the diet affects this parameter. The present study showed that the restoration to a NL diet in animals previously fed on the HL diet significantly reduced hepatic collagen, this reduction being greater in the olive oil group (with a diet rich in MUFAs) than in the sunflower oil group (with a diet rich in PUFAs). These results agree with the observations of Fernandez et al[31].
It is well known that hepatic steatosis raises the serum aminotransferase level[28,32,33], which is decreased after the treatment with hypocaloric diets[5,32]. Our results showed that treatment with a NL diet (olive or sunflower oil) could reduce the aminotransferase level. However, the decrease was more significant in the olive oil group. Since sunflower oil is more susceptible to oxidative stress due to its PUFA content, its hepatic recovery ability is presumably lower. Polavarapu et al[34]. and Grattagliano et al[35]. found that ALT activity increases in alcoholic subjects fed on high PUFAs diets, which also supports our results.
In short, the results of the present work indicate that a balanced diet enriched in olive oil contributes to the recovery from hepatic steatosis.
The authors thank Mr. David Nesbitt for his editorial help.
| 1. | Del Moral ML, Esteban FJ, Torres MI, Camacho MV, Hernandez R, Jimenez A, Aránega A, Pedrosa JA, Peinado MA. High-fat sunflower and olive oil diets affect serum lipid levels in steatotic rat liver differently. J Nutr Sci Vitaminol (Tokyo). 1997;43:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Scheen AJ, Luyckx FH. Metabolic syndrome: definitions and epidemiological data. Rev Med Liege. 2002;58:479-484. [PubMed] |
| 3. | Keegan A, Martini R, Batey R. Ethanol-related liver injury in the rat: a model of steatosis, inflammation and pericentral fibrosis. J Hepatol. 1995;23:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Esteban FJ, Sánchez-López AM, Del Moral ML, Camacho MV, Hernández R, Jiménez A, Pedrosa JA, Peinado MA. Effect of thioacetamide and dexamethasone on serum lipids in rats fed on high-fat sunflower or olive oil diets. J Nutr Sci Vitaminol (Tokyo). 1999;45:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Fan JG, Zhong L, Xu ZJ, Tia LY, Ding XD, Li MS, Wang GL. Effects of low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2003;9:2045-2049. [PubMed] |
| 6. | Cha YS, Sachan DS. Opposite effects of dietary saturated and unsaturated fatty acids on ethanol-pharmacokinetics, triglycerides and carnitines. J Am Coll Nutr. 1994;13:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Ramadori G, Rieder H, Theiss F, Meyer zum Büschenfelde KH. Fat-storing (Ito) cells of rat liver synthesize and secrete apolipoproteins: comparison with hepatocytes. Gastroenterology. 1989;97:163-172. [PubMed] |
| 8. | Burt AD, Le Bail B, Balabaud C, Bioulac-Sage P. Morphologic investigation of sinusoidal cells. Semin Liver Dis. 1993;13:21-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ramadori G. [Pathogenesis of liver fibrosis. Synthesis of collagen and non-collagen proteins in cell culture and in vivo]. Z Gastroenterol. 1992;30 Suppl 1:17-20. [PubMed] |
| 10. | Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39-S45. [PubMed] |
| 11. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Niki T, De Bleser PJ, Xu G, Van Den Berg K, Wisse E, Geerts A. Comparison of glial fibrillary acidic protein and desmin staining in normal and CCl4-induced fibrotic rat livers. Hepatology. 1996;23:1538-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Geerts A, Bouwens L, Wisse E. Ultrastructure and function of hepatic fat-storing and pit cells. J Electron Microsc Tech. 1990;14:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193-203. [PubMed] |
| 15. | Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Akiyoshi H, Terada T. Centrilobular and perisinusoidal fibrosis in experimental congestive liver in the rat. J Hepatol. 1999;30:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 1980;66:303-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 369] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271-277. [PubMed] |
| 19. | Burt AD, Robertson JL, Heir J, MacSween RN. Desmin-containing stellate cells in rat liver; distribution in normal animals and response to experimental acute liver injury. J Pathol. 1986;150:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Buniatian G, Hamprecht B, Gebhardt R. Glial fibrillary acidic protein as a marker of perisinusoidal stellate cells that can distinguish between the normal and myofibroblast-like phenotypes. Biol Cell. 1996;87:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Jimenez W, Parés A, Caballería J, Heredia D, Bruguera M, Torres M, Rojkind M, Rodés J. Measurement of fibrosis in needle liver biopsies: evaluation of a colorimetric method. Hepatology. 1985;5:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1017] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 23. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 159047] [Article Influence: 3180.9] [Reference Citation Analysis (1)] |
| 24. | Tsukamoto H, Cheng S, Blaner WS. Effects of dietary polyunsaturated fat on ethanol-induced Ito cell activation. Am J Physiol. 1996;270:G581-G586. [PubMed] |
| 25. | Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401-1410. [PubMed] |
| 26. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 406] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 27. | Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 406] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] |
| 30. | Szende B, Timár F, Hargitai B. Olive oil decreases liver damage in rats caused by carbon tetrachloride (CCl4). Exp Toxicol Pathol. 1994;46:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Fernández I, Torres I, Moreira E, Fontana L, Gil A, Rios A. Influence of administration of long-chain polyunsaturated fatty acids on process of histological recovery in liver cirrhosis produced by oral intake of thioacetamide. Dig Dis Sci. 1996;41:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Foster KJ, Griffith AH, Dewbury K, Price CP, Wright R. Liver disease in patients with diabetes mellitus. Postgrad Med J. 1980;56:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Nomura F, Ohnishi K, Ochiai T, Okuda K. Obesity-related nonalcoholic fatty liver: CT features and follow-up studies after low-calorie diet. Radiology. 1987;162:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, Nanji AA. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 221] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Grattagliano I, Palmieri VO, Palasciano G. Hepatotoxicity of polyunsaturated fatty acids in alcohol abuser. J Hepatol. 2002;37:291-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Co-first-authors: Raquel Hernández and MªÁngeles Peinado
Co-correspondent: MªÁngeles Peinado
Science Editor Wang XL and Guo SY Language Editor Elsevier HK