Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7466
Revised: June 1, 2005
Accepted: June 2, 2005
Published online: December 21, 2005
AIM: To investigate the influence of fish oil enriched enteral diet on intestinal tissues taken from Crohn’s disease (CD), ulcerative colitis (UC) and non-inflamed non-IBD control patients in vitro.
METHODS: Colonoscopic biopsies from patients with active CD (n = 4), active UC (n = 7), and non-inflamed non-IBD control patients (n = 4) were incubated (three dilutions of 1:20, 1:10, and 1:5) with Waymouth’s culture medium and enteral elemental diet (EO28, SHS, Liverpool, UK) modified in the fatty acid composition with fish oil (EF) in an organ culture system for 24 h. In each experimental set-up, incubation with Waymouth's medium alone as control was included. Tissue viability was assessed by adding bromodeoxyuridine (BrdU) to the culture fluid and immunohistochemically staining for BrdU uptake. Cytokine ratio of IL-1ra/IL-1β (low ratio indicative of inflammation) and production of those cytokines as a percentage of medium control were assayed in the culture supernatant.
RESULTS: Incubation of CD-affected tissue with EF (1:20, 1:10, and 1:5) modestly and non-significantly increased IL-1ra/IL-1β ratio as compared with medium control (CD 39.1±16.1; 26.5±7.8, 47.1±16.8 vs control 13.0±2.2), but incubation of UC-affected tissues increased IL-1ra/IL-1β ratio significantly in all three dilutions (UC 69.1±32.2, P<0.05; 76.1±36.4, P = 0.05; 84.5±37.3, P<0.02; vs control 10.2±3.7). Incubation of non-inflamed non-IBD control tissue did not increase the IL-1ra/IL-1β ratio in any dilution compared to medium control (69.3±47.0, 54.1±30.6, 79.4±34.0 vs control 76.1±37.3). Average percentage production of IL-1β indexed against medium control was significantly less in UC after EF incubation as compared with CD (UC 24.0±4.8 vs CD 51.8±8.1; P<0.05). Average percentage production of IL-1ra was markedly higher in UC (135.9±3.4) than that in control patients (36.5±4.3) (P<0.0001).
CONCLUSION: IBD tissues, after incubation with elemental diet modified in its fatty acid composition with fish oil, show an increase in IL-1ra /IL-1β cytokine ratio. This effect of ω-3 fatty acid modulation is significantly more marked in UC compared with CD and is accompanied by both a reduction of IL-1β and increase of IL-1ra. The positive direct anti-inflammatory effect of elemental diet with fish oil in tissue affected with UC suggests dietary treatment of UC may be possible.
- Citation: Meister D, Ghosh S. Effect of fish oil enriched enteral diet on inflammatory bowel disease tissues in organ culture: Differential effects on ulcerative colitis and Crohn’s disease. World J Gastroenterol 2005; 11(47): 7466-7472
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7466
Enteral defined formula nutritional therapy is currently an option only in Crohn’s disease (CD), and this may result in an improvement in clinical inflammatory parameters[1], histological appearance[2], and disease activity[3]. Hypotheses about how elemental diet might work include withdrawal of food antigens, decrease and alteration in luminal bacteria, reduction in pancreatic and biliary secretion, bowel rest (or medical bypass), alteration of dietary lipids and improvement in nutritional status[3]. Increased production of IL-1β during active inflammatory bowel disease (IBD)[4] and an imbalance of IL-1β and its receptor antagonist (IL-1ra) are now well-known features of IBD[5-7], and ratio of anti-inflammatory to pro-inflammatory cytokines IL-1ra/IL-1β may be used as an index of inflammatory status. The possible influence and modulation of cytokine balance by dietary fatty acids are of interest and might have clinical implication[8,9].
The main fatty acids of fish oil are the long-chain-polyunsaturated fatty acids (FA) of eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C20:6 ω-3). The use of these FA in inflammatory conditions has been explored in other inflammatory disorders, such as rheumatoid arthritis[10,11] and inflammatory skin disorders[12,13]. In IBD, especially in ulcerative colitis (UC), treatment with fish oil preparations may have a better impact than in CD, though this is still controversial. Studies have shown a decrease in clinical activity and some morphological improvements in UC but no change was observed in CD after 7-mo treatment with 3.2 g of ω-3 FA per day in active IBD[14]. Stenson et al[15]. reported the effect of oral fish oil treatment in UC in a double-blind placebo controlled crossover trial of 18 patients. Fish oil supplementation resulted in a significant decrease in rectal dialysate of LTB4, and significant improvement in the histological appearance of the colon. Furthermore, they reported a significant increase in body weight, averaging 1.74 kg, as compared to controls who had no change in body weight[15]. Clinical improvement in 11 patients with mild to moderate UC after ingestion of ω-3 FA was reported by Aslan and Triadafilopoulos[16]. Six months of supplementation with ω-3 FA in patients with proctocolitis resulted in a significant improvement in sigmoidoscopic and histologic appearance in the distal colon. Blood analysis showed a decreased natural killer (NK) cell activity and markedly reduced LTB4 serum levels, which correlated with a decrease in IL-2[17,18]. In contrast, Hawthorne et al[19]. reported only limited clinical benefit despite increased synthesis of LTB5 and suppression of LTB4 in ω-3-supplemented UC patients.
These studies, although controversial and differing in the delivery system and dose, demonstrate that dietary modulation via FA, especially of ω-3 and ω-6, can modulate and influence the inflammatory mediator production and response in IBD.
A theoretical advantage of dietary intake of ω-3 FA is that these FA do not have to go through the processes of elongation and saturation, and can therefore be immediately incorporated into the plasma membrane.
The aim of this study was to investigate the direct effect of fish oil containing enteral diet in vitro on cytokine response of IL-1ra/IL-1β ratio and on cytokine production as a percentage of medium control in tissue affected with CD, UC, and non-inflamed non-IBD control patients. Such in vitro experiments, though reductionist may provide indirect evidence of the alteration in inflammatory profile after the ingestion of defined formulae diet, which rapidly changes tissue levels of nutrients. In CD, it is clear that local effect cannot explain the benefit of such therapy in distal ileum and colon as most of elemental diet is absorbed in proximal small intestine. We specifically aimed to explore whether such modification of enteral diet used in CD might show evidence of efficacy in UC.
Fifteen patients were included in this study. Four patients with CD (mean age 41±14 years), seven patients with UC (mean age 56±6 years) and four patients as non-inflamed non-IBD control group (mean age 36±9 years). The latter group included patients with a final diagnosis of irritable bowel syndrome. The patients attended routine colonoscopy and gave consent to obtain additional biopsies along with the biopsies taken for clinical management. Ethical approval was obtained from Research Ethics Committee.
Biopsies were taken from the following anatomic areas: in CD (2 sigmoid, 1 rectum, 1 ileocolonic anastomosis), UC (5 sigmoid, 2 rectum) and control group (4 sigmoid). Disease duration for IBD patients was as follows: (1) 1-3 years (1 CD, 2 UC); (2) 4-6 years (1 CD, 1 UC); and (3) >6 years (2 CD, 4 UC). Medications at the time of biopsy were (1) in CD with azathioprine (n = 2), steroids and azathioprine (n = 1), steroids and 5-ASA (n = 1) and (2) in UC with steroids (n = 1), azathioprine (n = 1), 5-ASA (n = 3).
The biopsies in IBD patients were taken from macroscopically affected areas but avoiding frankly ulcerated areas, and histologically confirmed active inflammation and the diagnosis of IBD. The biopsies in control patients showed no evidence of inflammation. All biopsies were taken with identical biopsy forceps by the same surgeon (SG) to ensure uniform size of the biopsies.
Biopsies obtained from endoscopy were incubated for 24 h with elemental diet EO28 (SHS, Liverpool, UK), specially modified in its FA composition for fish oil (EF), as previously described[20]. Tables 1 and 2 show the details of diet composition regarding general composition of diet and FA profile, respectively. Briefly, enteral diet was diluted in distilled water in three dilutions of 1:5, 1:10, and 1:20 with Waymouth’s medium (MB752/1, Flow-labs 12-52-54). The medium was supplemented with 100 mL/L heat-inactivated fetal calf serum (Ako Tech), 200 mmol/mL L-glutamine (Gibco 43-8030), 300 μg/mL ascorbic acid (Sigma A 4544), 0.45 μg/mL ferrous sulfate (Sigma F 8633), 50 μg/mL gentamycin (Flow-labs 16-760-45), 5 000 IU/mL penicillin and 5 000 μg/mL streptomycin (Flow-labs 16-700-49).
| Nutrient | Per 100 g powder |
| Energy (kcal) | 443 |
| Protein equivalent (g) | 12.5 |
| Carbohydrate (g) | 60 |
| Fat (g) | 17.45 |
| Sodium (mg) | 305 |
| Potassium (mg) | 466 |
| Chloride (mg) | 333 |
| Calcium (mg) | 187.5 |
| Phosphorus (mg) | 200 |
| Magnesium (mg) | 81.6 |
| Iron (mg) | 4.2 |
| Zinc (mg) | 4.2 |
| Iodine (μg) | 33.3 |
| Manganese (mg) | 0.6 |
| Copper (mg) | 0.4 |
| Molybdenum (μg) | 33.3 |
| Selenium (μg) | 15 |
| Chromium (μg) | 15 |
| Vitamin A (μg) | 330 |
| Vitamin E (mg) | 8.3 |
| Vitamin C (mg) | 28.3 |
| Thiamine (mg) | 0.6 |
| Riboflavin (mg) | 0.6 |
| Pyridoxine (mg) | 0.8 |
| Niacin (mg) | 4.2 |
| Pantothenic acid (mg) | 2 |
| Inositol (mg) | 9.2 |
| Choline (mg) | 91.6 |
| Vitamin D (μg) | 1.9 |
| Vitamin B12 (μg) | 1.8 |
| Biotin (μg) | 58.3 |
| Vitamin K (μg) | 25 |
| Fatty acid g/100 g fat | Theoretical FA profilein % for fish oil (total 17.65 g) |
| C14:0 | 6.6 |
| C16:0 | 13.67 |
| C16:1 | 8.32 |
| C18:0 | 2.58 |
| C18:1 | 10.33 |
| C18:2 | 3.44 |
| C18:3 | 0.57 |
| C20:1 | 1.34 |
| C20:4 | 1.62 |
| C20:5 ω-3 | 18 |
| C22:5 ω-3 | 17.3 |
| C22:5 ω-6 | 1.91 |
| C22:6 ω-3 | 7.27 |
From each patient, one biopsy was always incubated with only medium as control. Tissue viability over culture period was confirmed by adding 10 μL/mL BrdU (Sigma B5002) to the culture fluid. After the culture period, supernatant was stored and frozen at -70 °C for immunoassay and tissue was processed for BrdU immunohistochemistry and validated as described previously[20,21]. All explants were examined in a blinded fashion under a light microscope (100× and 250×). The explants were considered viable only if the morphology of the tissue was considered intact with well-defined crypts, epithelial surface and adequate and strong uptake of BrdU with labeled cells in horizontal and vertical crypts. Formal scoring to determine labeling index was not performed as the focus of this study was not on colonic epithelial proliferation.
After 24 h incubation, IL-1ra and IL-1β were measured in culture supernatants by ELISA using matched antibody pairs (R&D Systems, UK) as previously described[20]. Capture antibody was supplied by R&D Systems (IL-1ra MAB280, IL-1β MAB601, R&D Systems). Standard proteins for IL-1ra (280-RA-010) and for IL-1β (201-LB-005) were also supplied by R&D Systems. Biotinylated detection antibodies, IL-1ra (BAF280) and IL-1β (BAF201), were purchased from R&D Systems. Streptavidin-horse-radish peroxidase conjugate (Zymed Laboratories, UK) and substrate TMB (R&D Systems) were used for IL-1ra and OPD substrate for IL-1β. Plates were read at 450 nm for IL-1ra, and at 490 nm for IL-1β.
Preliminary experiments showed that cytokine recoveries after incubation in defined formula diet for both IL-1ra and IL-1β were satisfactory. Formula diet spiked at low concentration (100 pg/mL) showed that more than 50% of IL-1ra cytokine was detected over the entire 24-h incubation period and that in diet spiked with IL-1ra at high concentration (1 000 pg/mL), a steady decrease of detectable cytokine was measured from 100% recovery at 0 h to 52% after 24 h.
Defined formula diet spiked with IL-1β at low concentration (10 pg/mL) showed that IL-1β was 100% detectable at all time points (0, 3, 6, and 24 h), and at a high concentration (100 pg/mL), 100% recovery at time points 0, 3, and 6 h but only 60% at 24 h.
As IL-1ra and IL-1β were continually secreted into the supernatant, the actual recovery in these experiments is likely to be higher than the recovery 24 h after spiking with cytokines. Standard curves in EF diet for IL-1ra and IL-1β showed no evidence of interference with the ELISA as compared with curves performed with medium alone, which again confirmed the reproducibility of the obtained data.
Statistics was performed using the Minitab 10 program. Mann-Whitney test and one-way analysis of variance (ANOVA) were performed to detect differences between diet incubations. Differences were considered significant at P<0.05. Data for IL-1ra/IL-1β ratio were compared with medium control, if not otherwise stated and presented as mean values and standard error of the mean (SEM). The numbers were too low to perform any subgroup analysis depending on the duration of disease or concurrent medications.
A total of 59 biopsies were included in the final analysis as follows: (1) CD medium (n = 4), EF diet (n = 12); (2) UC medium (n = 6), EF diet (n = 21); and (3) control patients’ medium (n = 4), EF diets (n = 12). All the explants fulfilled the criteria of intact morphology and BrdU uptake in order to be included in this final analysis. BrdU-labeled cells occurred in vertical crypts at the base of the crypts and along the sides of the crypts and intact epithelium.
IL-1ra/IL-1β ratio is indicative of the inflammatory status of the tissue and is independent of the tissue weight. We have reported this ratio previously in organ culture of IBD-affected tissue as sensitive to changes after incubation with defined formula diets of different compositions[20]. As the tissue is inflamed, the ratio tends to decrease and vice versa.
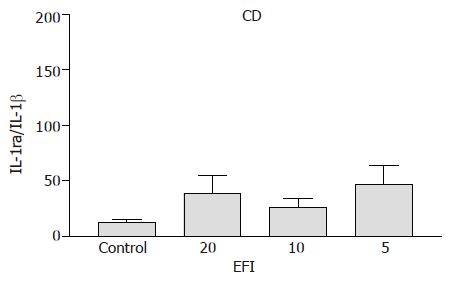
Crohn’s disease A trend towards an increase in IL-1ra/IL-1β ratio was observed for 1:20, 1:10, and 1:5 dilutions of EFI (39.1±16.1; 26.5±7.8; 47.1±16.8 vs medium control 13.0±2.2), respectively, but this did not reach statistical significance (Figure 1).
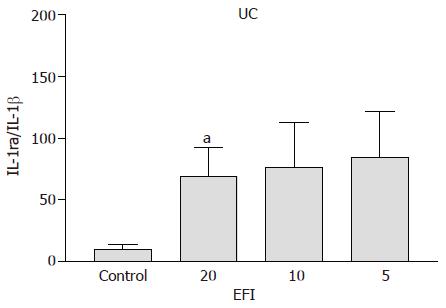
Ulcerative colitis A significant increase in IL-1ra/IL-1β ratio for 1:20, 1:10, and 1:5 dilutions of EFI (69.1±32.2, P<0.05; 76.1±36.4, P = 0.05; 84.5±37.3, P<0.02 vs medium control 10.2±3.7) was noted (Figure 2). The ratio was similar in CD and UC patients for medium control alone and significantly lower than non-inflamed non-IBD control patients (shown below), confirming the inflammatory status of these tissues.
Control patients No increase in the IL-1ra/IL-1β ratio was detectable for 1:20, 1:10, 1:5 dilutions of EFI (69.3±47.0; 54.1±30.6; 79.4±34.0 vs 76.1±37.3). This is mainly due to the fact that the ratio in the medium control was high (as expected in non-inflamed tissues) and this did not increase any further with EF incubation.
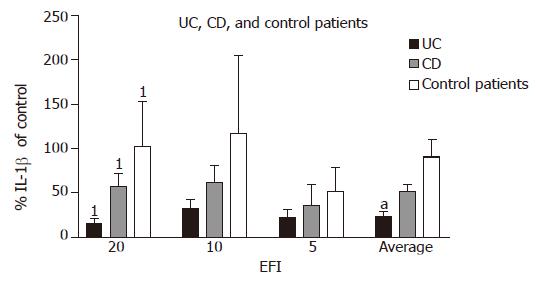
Interleukin-1β Percentage production of IL-1β in UC (as a proportion of medium control) was lower at all the three dilutions as compared with CD and this reached statistical significance for 1:20 dilution (UC 32.6±9.8% vs CD 62.1±19.1%; P = 0.02). The average production (in all three dilutions of EF) of IL-1β was significantly more suppressed in UC than that in CD (24.0±4.8% vs 51.8±8.1%; P<0.05). In UC patients, the decrease in production of IL-1β was also significant as compared with control patients at 1:20 dilution (32.6±9.8% vs 117.3±87.3%; P = 0.02). In control patients, there was a little suppression of IL-1β after incubation with EF except at the highest concentration of 1:5 (Figure 3).
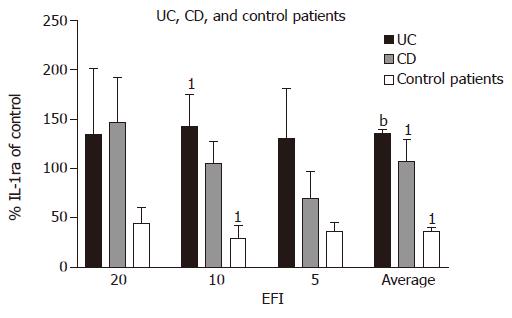
Interleukin1-receptor antagonist In UC patients, secretion of IL-1ra (as percentage of medium control) tends to be higher when compared with CD patients at 1:10 and 1:5 dilutions (UC 142.3±33.2% and 130.5±50.3% vs CD 104.9±13.2% and 70.1±35.9%), respectively. Compared with control patient, secretion of IL-1ra was increased for 1:20, 1:10, and 1:5 dilutions of EF [UC (134.9±66.1%; 142.3±33.2%; 130.5±50.3%) vs control (44.1±16.2%; 29.4±13.2%; 35.9±10.1%)], respectively, and average production of IL-1ra for all dilutions was significantly increased (UC 135.9±3.4% vs controls 36.5±4.3%; P<0.0001).
In CD patients, there was an increase in IL-1ra secretion (as percentage of medium control) at all three dilutions as compared with control patients (in whom IL-1ra decreased) (CD 146.3±45.6%; 104.9±13.2%; 70.1±35.9%; control 44.1±16.2%; 29.4±13.2%; 35.9±10.1%), reaching significance at 1:10 dilution (P = 0.03). The average increase in IL-1ra in CD patients for all three dilutions was significant vs control patients (CD 107.1±22.0% vs 36.5±4.3%; P = 0.03).
In control patients, IL-1ra production tends to decrease after incubation with EF as a proportion of medium control, unlike CD and UC (Figure 4).
Fish oil preparations in the form of gelatin-coated fish oil capsules have been used to treat UC in several studies. Hawthorne et al[16,19]. Stenson et al[15]. and Loeschke et al[22]. investigated (in placebo-controlled trials) the influence of ω-3 FA, EPA and DHA derived from fish oil on prostaglandin and leukotriene synthesis in patients with UC. These studies illustrated that treatment with fish oil led to a corticosteroid sparing effect and a reduction in LTB4 measured in colonic mucosa, rectal dialysate and suppression in synthesis of LTB4 in peripheral blood neutrophils[15,16,19]. Contrary to these studies, we investigated, in the in vitro organ culture model, the direct influence of fish oil containing enteral diet (EFI) on pro- and anti-inflammatory cytokine production assayed in the culture supernatant. We found a significant increase in IL-1ra/IL-1β ratio in UC patients as compared with medium control as a result of enteral diet-fish oil incubation. This increase was not found in non-inflamed non-IBD control patients and was less marked in CD patients. This might be as a result of an increased production of IL-1ra and decreased production of IL-1β. Average percentage of IL-1ra release in UC patients was higher than in CD and significantly higher compared with control patients after the incubation with EF. The percentage of IL-1β production of medium control showed, in all three dilutions of EF, a more marked decrease in UC compared with CD and control patients. In this model of intestinal tissue incubation for 24 h period, it would appear that enteral diets containing ω-3 FA have a direct in vivo anti-inflammatory and immunomodulatory impact, especially in UC. It seems that fish oil not only increases IL-1ra production, but also inhibits the production of IL-1β. This modulation of cytokine response by fish oil containing defined formula diet EF in UC patients is possibly due to the modified fatty acid profile with increased ω-3 FA. This preferentially favors the production of prostaglandins and thromboxanes of series 3 and leukotrienes of series 5, with decreased pro-inflammatory properties.
Mahida et al[23]. also used a similar organ culture model similar to that used by us in their study. Colonic biopsies of patients with UC were incubated with and without 5-aminosalicylic acid (5-ASA). Significantly higher IL-1β was detected in inflamed colonic biopsies compared to normal controls. After the incubation with 5-ASA, there was a significant dose-dependent decrease of IL-1β production as a percentage of controls. It also showed a significant correlation of IL-1β and TXB2 synthesis in culture medium of inflamed tissue as compared with controls. A significant decrease in LTB4 was found in inflamed tissue treated with 5-ASA. It is known that 5-ASA can inhibit the synthesis of PGE2, LTB4 and their products of the 5-lipoxygenase pathway. It is likely that these ω-3 FA affect the IL-1β synthesis either by altering the eicosanoid synthesis or by having a direct influence of FA on gene expression. The two major polyunsaturated fatty acids (PUFAs) in fish oil are EPA and DHA, which are also major PUFAs incorporated into the cell membrane. EPA is a substrate for eicosanoid production via cyclooxygenase pathway for PG3 and lipoxygenase pathway for TX3, both known as having less inflammatory properties. Watanabe et al[24]. showed that in LPS stimulated mice lymphocytes, where mice had been fed with beef tallow, the two fish oil FA, DHA and EPA, produced different IL-1β mRNA expression. Decreased production of IL-1β mRNA after EPA and DHA was significant in DHA vs tallow and slightly lower in EPA as compared with DHA. Production of PGE2 was significantly decreased in EPA and DHA, though DHA is not a precursor for eicosanoids[25]. As IL-1β mRNA response differed between EPA and DHA, though the PGE2 production was similar, it seems that the balance of EPA/DHA in cellular lipids might be important in affecting intracellular signaling through modifications of specific gene transcription[24]. FA in the cell are bound on fatty acid binding proteins (FABP), which exist in the cytosol and nucleus, in the form of fatty acid acyl-CoA (FA-CoA). The nuclear receptors peroxisome proliferator-activated receptors (PPARs) occur in three identified subtypes of α, β, and γ, which are regulated by FA. It is likely that some FA or their metabolites may act as hormones to control specific transcription factors. For example, PPARα is required for fish oil-mediated induction of mRNAAOX (mRNA - acyl-CoA oxidase). Nuclear transcription factor, NFκB, is required in genes, which are involved in pro-inflammatory responses, such as cyclooxygenase 2 and cytokines IL-1β and TNF-α. The effect of ω-3 FA might be attributed to the PPARα activation and the regulation of NFκB-mediated transcription[26]. As incubation with fish oil resulted in marked and divergent anti- and pro-inflammatory cytokine response, it is possible that ω-3 PUFA result in different cytokine production in various cell types[24]. It may also reflect a different response in IBD subtypes, as IL-1ra/IL-1β was not significantly increased in CD after incubation with EF. Furthermore, as EPA and DHA act differently in eicosanoid and IL-1β production, both of these ω-3 FA in fish oil may contribute to the anti-inflammatory effect of fish oil.
Biopsies incubated with EF, as a major source of ω-3 FA in the present study, have shown a significant positive response towards an anti-inflammatory effect only in UC. In the current literature, the immunomodulatory properties of fish oil manifests as a decrease of LTB4 or increased synthesis of LTB5[16,27]; however, a change in IL-1β mRNA expression after LPS stimulation in mouse spleen lymphocytes has also been reported[24]. The actual increase in IL-1ra/IL-1β ratio could be interpreted as a reaction of eicosanoid-modulated cytokine production through G proteins and/or FA-modulated gene transcription via transcription factor. Which of these factors may have the predominant impact on immunomodulatory properties of fish oil is not defined as yet. Several studies have demonstrated that DHA might be superior as compared with EPA in immunomodulatory activity[24,28]. This suggests that the effects of fish oil might be a response of FA-induced gene transcription, as eicosanoids are derivatives of dihomo-γ-linolenic acid (DLGA, 20:3 ω-6), arachidonic acid (AA C20:4 ω-6) and eicosapentaenoic acid (EPA C20:5 ω-3)[25] and not of docosahexaenoic acid (DHA C22:6 ω-3).
In this study, the results of the supernatant cytokine concentration were presented predominantly as a ratio of anti-inflammatory to pro-inflammatory cytokine (IL-1ra:IL-1β) as this was independent of the weights of the biopsies. The cytokine concentrations were not indexed against the protein or DNA content of the biopsies as the biopsies were processed for the uptake of BrdU and determination of tissue viability after 24 h incubation. The ratio of cytokines, which is independent of the explant weights, has been used in our previous publication on the effect of elemental diet alone[20]. Each individual set of biopsies from one patient was simultaneously put in organ culture at the different dilutions of EI as well as a medium control, within 10 min of collection of the biopsies. All biopsies in this study were taken with identical biopsy forceps by the same surgeon, and therefore were uniform in size. This enabled representation of the results of EF incubation as a proportion of the cytokine concentration produced by incubation in medium control alone without any added defined formula diet. The results of the changes in cytokine ratios were consistent with the changes in individual cytokines indexed as a proportion of the medium control incubation. All explants included in the analysis were considered viable by morphology and BrdU uptake.
We have previously reported on the results of incubation of IBD (CD and UC) affected explants with elemental diet (EO-28) as well as modified elemental diet with altered nitrogen source (casein and whey). These experiments were identical in design to the present study and showed direct anti-inflammatory effect of elemental diet on CD but not UC-affected tissues. This anti-inflammatory effect was manifested by a rise in IL-1ra/IL-1β ratio, and persisted when elemental diet was modified with casein and whey. In this study, we have showed that when elemental diet was modified by fish oil, but the rest of its composition including amino acids were kept unaltered, the anti-inflammatory effect could be observed on UC-affected tissues. It is striking that by modifying the composition of elemental diet, the anti-inflammatory effect could be made to be more pronounced in CD (conventional EO-28) or in UC patients (fish oil modified EO-28). This raises the possibility that such modifications could provide the option of nutritional therapy for UC patients, and again illustrates the difference between CD and UC patients in nutritional modulation and immunology.
The in vitro model is reductionist and local delivery of fish oil could be a problem by the oral delivery of fish oil modified enteral diet. Rectal delivery or enteric coated preparations would be necessary. Systemic effects of oral delivery are also possible and almost certainly mediate part of the beneficial effects of elemental diet especially on terminal ileum and colon. Nevertheless, these experiments suggest that enteral defined formula diet may have direct anti-inflammatory effects, and the composition may determine whether it is effective in CD or UC patients. Absorption after oral administration will alter the nutrient milieu of cells and possibly their inflammatory response.
In conclusion, we have shown a positive anti-inflammatory effect on tissues incubated with enteral diets modified with fish oil, which is significant only in UC, but much more modest in CD. This study provides a rationale for exploring dietary therapies for UC patients.
We thank SHS, Liverpool UK for supplying us with the specially prepared enteral diet modification and MC Aldhous for technical assistance.
| 1. | Rigaud D, Cosnes J, Le Quintrec Y, René E, Gendre JP, Mignon M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: elemental versus polymeric diet. Gut. 1991;32:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Fell JM, Paintin M, Arnaud-Battandier F, Beattie RM, Hollis A, Kitching P, Donnet-Hughes A, MacDonald TT, Walker-Smith JA. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn's disease. Aliment Pharmacol Ther. 2000;14:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 283] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 3. | Teahon K, Pearson M, Smith T, Bjarnason I. Alterations in nutritional status and disease activity during treatment of Crohn's disease with elemental diet. Scand J Gastroenterol. 1995;30:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990;31:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 319] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998;112:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Andus T, Daig R, Vogl D, Aschenbrenner E, Lock G, Hollerbach S, Köllinger M, Schölmerich J, Gross V. Imbalance of the interleukin 1 system in colonic mucosa--association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut. 1997;41:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995;154:2434-2440. [PubMed] |
| 8. | Grimble RF, Tappia PS. Modulation of pro-inflammatory cytokine biology by unsaturated fatty acids. Z Ernahrungswiss. 1998;37 Suppl 1:57-65. [PubMed] |
| 9. | Meydani SN, Dinarello CA. Influence of dietary fatty acids on cytokine production and its clinical implications. Nutr Clin Pract. 1993;8:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:349S-351S. [PubMed] |
| 11. | Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. 2000;71:352S-356S. [PubMed] |
| 12. | Ziboh VA, Miller CC, Cho Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: generation of antiinflammatory and antiproliferative metabolites. Am J Clin Nutr. 2000;71:361S-366S. [PubMed] |
| 13. | Burton JL. Dietary fatty acids and inflammatory skin disease. Lancet. 1989;1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Lorenz R, Weber PC, Szimnau P, Heldwein W, Strasser T, Loeschke K. Supplementation with n-3 fatty acids from fish oil in chronic inflammatory bowel disease--a randomized, placebo-controlled, double-blind cross-over trial. J Intern Med Suppl. 1989;731:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 109] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Stenson WF, Cort D, Rodgers J, Burakoff R, DeSchryver-Kecskemeti K, Gramlich TL, Beeken W. Dietary supplementation with fish oil in ulcerative colitis. Ann Intern Med. 1992;116:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 243] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Aslan A, Triadafilopoulos G. Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am J Gastroenterol. 1992;87:432-437. [PubMed] |
| 17. | Almallah YZ, Richardson S, O'Hanrahan T, Mowat NA, Brunt PW, Sinclair TS, Ewen S, Heys SD, Eremin O. Distal procto-colitis, natural cytotoxicity, and essential fatty acids. Am J Gastroenterol. 1998;93:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Almallah YZ, El-Tahir A, Heys SD, Richardson S, Eremin O. Distal procto-colitis and n-3 polyunsaturated fatty acids: the mechanism(s) of natural cytotoxicity inhibition. Eur J Clin Invest. 2000;30:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Hawthorne AB, Daneshmend TK, Hawkey CJ, Belluzzi A, Everitt SJ, Holmes GK, Malkinson C, Shaheen MZ, Willars JE. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut. 1992;33:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 196] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 20. | Meister D, Bode J, Shand A, Ghosh S. Anti-inflammatory effects of enteral diet components on Crohn's disease-affected tissues in vitro. Dig Liver Dis. 2002;34:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Wilson RG, Smith AN, Bird CC. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J Clin Pathol. 1990;43:744-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Loeschke K, Ueberschaer B, Pietsch A, Gruber E, Ewe K, Wiebecke B, Heldwein W, Lorenz R. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig Dis Sci. 1996;41:2087-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Mahida YR, Lamming CE, Gallagher A, Hawthorne AB, Hawkey CJ. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991;32:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Watanabe S, Katagiri K, Onozaki K, Hata N, Misawa Y, Hamazaki T, Okuyama H. Dietary docosahexaenoic acid but not eicosapentaenoic acid suppresses lipopolysaccharide-induced interleukin-1 beta mRNA induction in mouse spleen leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2000;62:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 437] [Article Influence: 16.2] [Reference Citation Analysis (3)] |
| 27. | Hillier K, Jewell R, Dorrell L, Smith CL. Incorporation of fatty acids from fish oil and olive oil into colonic mucosal lipids and effects upon eicosanoid synthesis in inflammatory bowel disease. Gut. 1991;32:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK