INTRODUCTION
Hepatocyte growth factor (HGF) is a multifunctional cytokine involved in proliferation, motility, and morphogenesis[1]. The effects of HGF are mediated through the tyrosine kinase receptor, c-Met[2]. HGF is reportedly the most potent mitogen for hepatocytes and acts as a trigger for liver regeneration[3,4]. Indeed, HGF knockout mice died in utero and the embryonic liver was reduced in size and had extensive loss of parenchymal cells[5]. In c-Met conditional knockout mice produced by using the Mx-cre transgene to introduce the mutation in the adult, liver regeneration after PH was impaired[6,7]. In c-Met conditional knockout mice produced by Cre/loxP-mediated gene targeting, recovery from necrosis induced by carbon tetrachloride (CCl4) was impaired[8]. These data indicate that the HGF/c-Met signaling pathway plays an important role in liver regeneration and repair.
HGF is a heterodimeric glycoprotein consisting of an α chain of 60 ku and a β chain of 30 ku[9]. The α chain has an N domain and four kringle domains. HGF mRNA can undergo alternative splicing to create a truncated isoform, NK2, which consists of an N domain and the first two kringle domains of HGF[10]. NK2 is also able to bind to c-Met with relatively high affinity and is thought to be an HGF antagonist of a variety of biological activities in vitro[1,11-13]. In vivo, it has been reported that NK2 acts as an antagonist of HGF in cellular proliferation and protective effect on CCl4 induced hepatotoxicity[14,15].
Here we have reported that NK2 inhibits hepatocyte proliferation and impairs liver regeneration after PH in our transgenic model.
MATERIALS AND METHODS
Animals
NK2 transgenic mice were generated on an albino FVB genetic background as previously described[14]. Expressions of human NK2 cDNA was under the control of the mouse metallothionein-1 (MT-1) promoter and associated locus control regions as previously described[16]. Wild-type (WT) mice were littermates of NK2 transgenic mice. All studies were performed using 6-wk-old female mice. PH, consisting of removal of the median and left lateral hepatic lobes, was performed as previously described[17]. Survival was observed with 21 NK2 transgenic mice and 23 WT mice over 10 d. Percentage of liver regeneration was defined as follows: [wet weight of regenerating liver/body weight (g/g mouse)]/[wet weight of original liver/body weight (g/g mouse)]×100%[18]. The animal experiments were conducted in compliance with the guidelines for animal care and use established by Gunma University Graduate School of Medicine.
Histology
Mice were injected with 50 μg/g of 5-bromo-2’-deoxyuridine (BrdU, Becton Dickinson, San Jose, CA, USA) 1 h before being killed. Livers were removed before and 48 h after PH. The tissues were fixed in 10% formalin, embedded in paraffin and stained with hematoxylin-eosin (H-E) and Oil-red-O. For immunohistochemistry, slides were stained with monoclonal antibody to BrdU. At 48 h after PH, the labeled hepatocyte nuclei were scored by counting 30 high-power light microscope fields (×1 000) for each animal.
Northern blot analysis
Total RNAs from liver tissues were prepared using TRIzol (Gibco BRL, Gaithersburg, MD, USA) according to the instructions provided by the manufacturer, and 20 μg of total RNA was loaded per lane onto a 1% agarose-formaldehyde gel and transferred to a nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). As described previously, transcripts of the NK2 transgene and endogenous c-Met were detected with a 2.2-kbp mouse HGF cDNA probe and a 1.5-kbp mouse c-Met cDNA probe, respectively[15]. The membrane was rehybridized with a mouse β-actin probe (generously provided by Kojima I) to control for RNA loading and transfer variation.
Immunoprecipitation (IP) and immunoblotting (IB)
Quantification of c-Met and c-Met tyrosine phosph-orylation was performed as described previously[19]. Briefly, frozen liver tissues were solubilized in RIPA buffer consisting of 50 mmol/L Tris (pH 7.4), 50 mmol/L NaCl, 1% Triton X-100, 5 mmol/L ethylenediaminetetraacetic acid, 10 mmol/L sodium PPi (Sigma, St. Louis, MO, USA), 50 mmol/L sodium fluoride (Sigma), 1 mmol/L sodium orthovanadate (Sigma), 1 mmol/L phenylmethylsulfonyl fluoride (Boehringer Mannheim, Mannheim, Germany), 10 μg/mL leupeptin (Boehringer Mannheim), 10 μg/mL pepstatin (Boehringer Mannheim, Tokyo, Japan) and 10 μg/mL aprotinin (Boehringer Mannheim). Protein concentration in the resulting lysates was determined using Albumin Standard (Pierce, Rockford, IL, USA) and equivalent amounts of lysate (200 μg) were incubated with anti c-Met antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h on ice. After the addition of GammaBind G Sepharose (Boehringer Mannheim) and washing in RIPA buffer, samples were fractionated on 10% polyacrylamide gels (Biocraft). After electrophoretic transfer to nitrocellulose membranes (Bio-Rad, Richmond, CA, USA), filters were blocked and then incubated with anti c-Met antibody overnight. c-Met was visualized by incubation with anti-goat antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, CA, USA) by using enhanced chemiluminescence (Santa Cruz Biotechnology, CA, USA) according to the instructions supplied by the manufacturer. Subsequently, filters were stripped with buffer consisting of 100 mmol/L 2-mercaptoethanol (Sigma), 2% sodium dodecyl sulfate, and 62.5 mmol/L Tris-HCl (pH 6.7) at 50 °C for 30 min. Filters were reblocked and incubated overnight with anti-phosphotyrosine (PY) antibody (Transduction Laboratories, Lexington, KY, USA) overnight. PY was visualized using an anti-rabbit antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, CA, USA) and enhanced chemiluminescence (Santa Cruz Biotechnology, CA, USA) according to the instructions obtained from the manufacturer.
Reverse transcriptase polymerase chain reaction (RT-PCR) assay
RT-PCR was used to measure the expression of liver TNF-α mRNA and liver IL-6 mRNA. Total RNAs from liver tissues were prepared using TRIzol as previously described[15]. cDNA was prepared from 1 μg of total RNA from each liver sample by the SuperScript Preamplification System for First Strand cDNA Synthesis kit (GIBCO BRL) according to the manufacturer’s instructions. The PCR reaction contained, in the same buffer as the reverse transcriptase reaction, cDNA corresponding to 50 ng of input RNA and 2.5 U AmpliTaq DNA polymerase. It was performed at 94 °C for 1 min, 55 °C for 1 min, 72 °C for 1.5 min for 35 cycles. Amplified products were electrophoresed in 2% agarose gels and stained with ethidium bromide. The primer pairs used were as follows[20]. TNF-α sense, 5’-AGCCCACGTCGTAGCAAACCACCAA-3’; antisense, 5’-ACACCCATTCCCTTCACAGAGCAAT-3’ (448-bp product size), IL-6 sense, 5’-TATGAAGTTCCTCTCTGCAA-3’; antisense, 5’-CTTTGTATCTCTGGAAGTTT-3’ (285-bp product size), β-actin sense, 5’-GCACCACACCTTCTACAATGAG-3’; antisense, 5’-AAATAGCACAGCCTGGATAGCAAC-3’ (150-bp product size).
Statistical analysis
All experimental data are shown as mean±SD. Cumulative survival was plotted by the Kaplan-Meier method, and the significance of differences was examined by the log-rank test. Differences in the index of BrdU-labeled hepatocytes and percentage of liver regeneration were determined by the Student’s t-test for each group. The level of significance for all statistical analyses was set at P<0.01.
RESULTS
Survival after PH
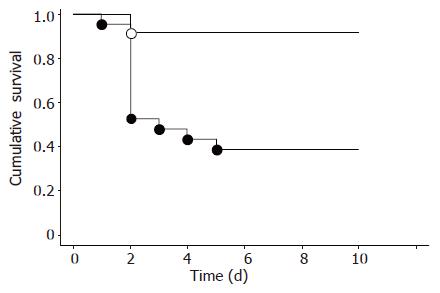
We examined the effect of NK2 on mortality of mice after PH (Figure 1). Ten of 21 NK2 transgenic mice (47.6%) died within 2 d. In contrast, 2 of 23 WT mice (8.7%) died within 2 d. Survival of NK2 transgenic mice after PH was significantly decreased compared with that of WT mice (P<0.01). Sham operation was performed using four mice per group. All four NK2 transgenic mice and all four WT mice survived until experiments were terminated after 10 d (data not shown).
Figure 1 Cumulative survival after PH.
Survival of WT mice (n = 23, open circles) and NK2 transgenic mice (n = 21, closed circles) after PH. The survival rate was significantly low in NK2 transgenic mice (bP<0.01 vs WT mice).
Morphological alteration in liver and hepatocyte proliferation after PH
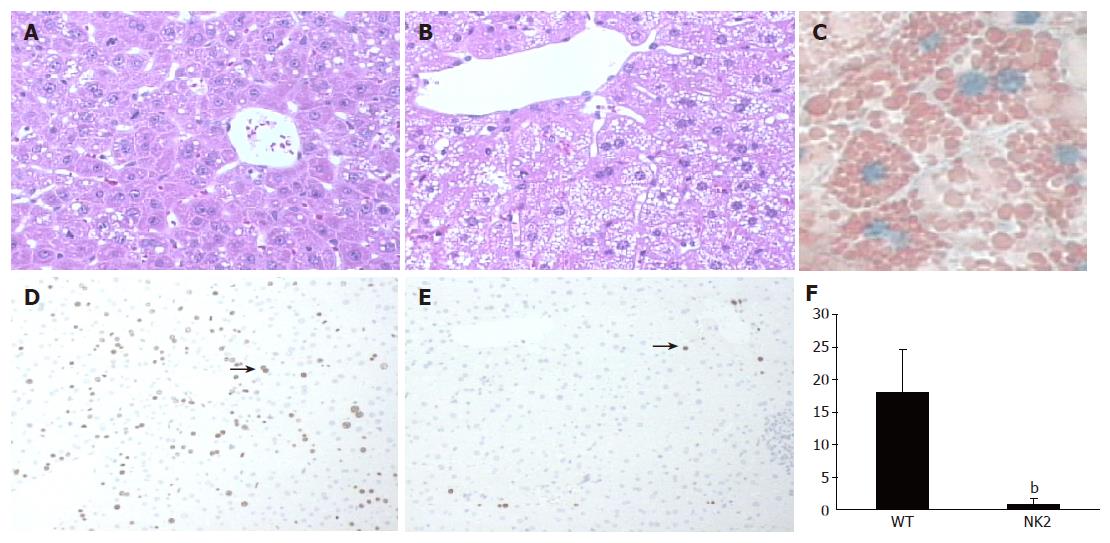
The liver structure of regenerating livers of WT mice after PH was normal (Figure 2A). NK2 transgenic mice that died during the first 48 h after PH had smooth and yellow livers. Histological examination revealed that livers of the dying NK2 transgenic mice contained a very large amount of intracellular small droplets at 48 h after PH (Figure 2B). These vesicles were readily identifiable as lipid by Oil-red-O stain (Figure 2C). BrdU staining was performed to analyze hepatocyte proliferation of each mouse at 48 h after PH (Figures 2D and E). The labeling index of the dying NK2 transgenic hepatocytes was significantly decreased relative to that of WT at 48 h after PH (WT, 18.0±6.6; dying NK2, 0.8±1.0, P<0.01) (Figure 2F). On the other hand, the percentage of liver regeneration of healthy NK2 transgenic mice and that of WT mice at 48 h after PH were 69.0±8.6% (n = 5) and 66.1±5.0% (n = 4), respectively (P = 0.59).
Figure 2 Liver regeneration and proliferation of hepatocytes after PH.
H-E stain of WT mouse liver (A) and NK2 transgenic mouse liver (B) at 48 h after PH. NK2 transgenic mouse liver contained a large amount of intracellular small droplets. Magnification, ×200. Oil-red-O stain of NK2 transgenic mouse liver (C) at 48 h after PH. Note the presence of many small lipid droplets in hepatocytes. Magnification, ×400. BrdU stain of WT mouse liver (D) and NK2 transgenic mouse liver (E) at 48 h after PH. The arrows show hepatocytes undergoing DNA synthesis. Magnification, ×100. The BrdU labeling index of WT mice and NK2 transgenic mice at 48 h after PH (n = 30 per group). (F) Data are mean±SD of the mean. Hepatocyte proliferation was significantly reduced in NK2 transgenic mouse liver (bP<0.01 vs WT mouse liver).
Expression of the transgene, endogenous c-Met and activation of c-Met in the liver after PH
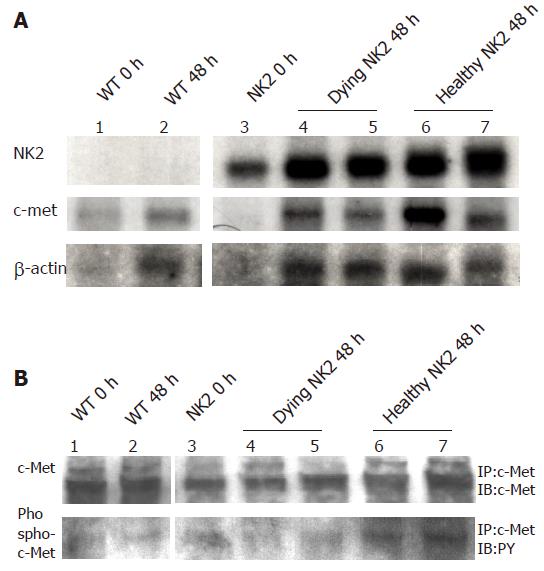
High expression of transgene was detected at each time point in NK2 transgenic livers (Figure 3A). There was no difference of endogenous c-Met expression among WT mice, dying NK2 transgenic mice and healthy NK2 transgenic mice (Figure 3A). We next investigated the expression and phosphorylation of c-Met protein. The levels of c-Met protein were in accordance with the c-Met transcription findings and there was no difference of tyrosine phosphorylation of c-Met among the WT mice, dying NK2 transgenic mice and healthy NK2 transgenic mice (Figure 3B).
Figure 3 Expressions of RNAs and proteins of transgene and c-Met after PH.
RNA expression of transgenic NK2 and c-Met, and c-Met protein levels and activity in livers from WT and NK2 transgenic mice after PH. Lane 1, WT mouse before PH; lane 2, WT mouse at 48 h after PH; lane 3, NK2 transgenic mouse before PH; lanes 4 and 5, dying NK2 transgenic mice at 48 h after PH; lanes 6 and 7, healthy NK2 transgenic mice at 48 h after PH. (A) Each RNA sample was subjected to Northern blot analysis. Expression levels of all transgenes were high at each time point and endogeneous c-Met expression was unaltered in each group. β-actin RNA expression was also examined as a control. (B) Each protein sample was subjected to IP and then IB with an anti c-Met antibody or an anti PY antibody. c-Met expression and the kinase activity were unaltered in each group.
Hepatic expression of TNF-α and IL-6 after PH
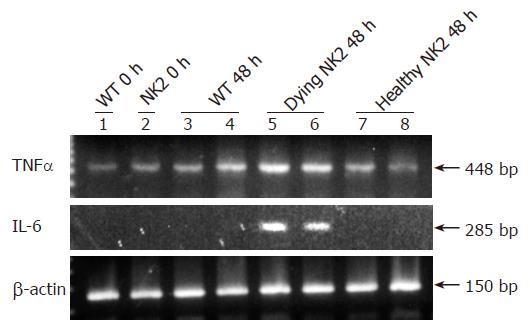
We next examined the expressions of TNF-α and IL-6 mRNAs before PH and 48 h after PH in the livers of WT mice, dying NK2 transgenic mice and healthy NK2 transgenic mice by RT-PCR (Figure 4). TNF-α mRNA was increased in the livers of the dying NK2 at 48 h after PH. In contrast, the level of TNF-α mRNA expression at that point was similar to that before PH in WT mice and healthy NK2 transgenic mice. IL-6 mRNA was detected in the livers of the dying NK2 at 48 h after PH but not detected in WT mice and healthy NK2 transgenic mice at that point.
Figure 4 RNA expression of TNF-α and IL-6 after PH.
TNF-α mRNA and IL-6 mRNA expression in livers of WT mice and NK2 transgenic mice. Lane 1, WT mouse before PH; lane 2, NK2 transgenic mouse before PH; lanes 3 and 4, WT mice at 48 h after PH; lanes 5 and 6, dying NK2 transgenic mice at 48 h after PH; lanes 7 and 8, healthy NK2 transgenic mice at 48 h after PH. The expression of TNF-α mRNA was increased in NK2 transgenic mice that died at 48 h after PH. IL-6 mRNA expression was detected in NK2 transgenic mice that died at 48 h after PH. β-actin mRNA expression was also examined as a control.
DISCUSSION
HGF is a potent mitogen for hepatocytes and appears to act on hepatocytes during liver regeneration after PH[3]. Indeed, HGF stimulates liver regeneration after PH in HGF transgenic mice[18,21]. NK2 is a naturally occurring HGF alternative splice variant and acts as an antagonist of HGF in vitro[1]. However, very little is known about the in vivo role of NK2 in the modulation of HGF activity. When NK2 transgenic mice, created using a mouse MT-1 promoter and associated locus control regions, were treated with zinc sulfate in water, a small reduction in liver size was observed[14]. This finding indicated that NK2 was able to inhibit endogenous HGF-mediated hepatocyte proliferation in vivo. Recently, we have demonstrated that overexpression of NK2 does not inhibit hepatocyte proliferation after liver damage induced by CCl4 administration using NK2 transgenic mice[15]. In this study, we have investigated the effects of NK2 on liver regeneration after PH using NK2 transgenic mice. After PH, about half of the NK2 transgenic mice died within 2 d, whereas almost all WT mice survived that period. NK2 transgenic mice did not exhibit overt abnormal phenotypes, including the liver[15]. However, the NK2 transgenic mice that died after PH showed massive intracellular accumulation of lipid in hepatocytes at 48 h after PH. Moreover, the BrdU leveling index of hepatocytes in NK2 transgenic mice at 48 h after PH demonstrated their poor capacity to enter the S phase. Because DNA replication after PH starts at 20 to 34 h and reaches a peak at 36 to 44 h in mice[22,23], these results indicate that overexpression of NK2 inhibited DNA replication of hepatocytes after PH. The plasma HGF level was reported to peak at 48 h after PH[24]. Hepatic expression of the NK2 transgene transcript was very high at each time point after PH. This suggested that overexpression of NK2 inhibited the proliferative effect of endogeneous HGF after PH. On the other hand, the expression of endogenous c-Met RNA and c-Met protein, and phosphorylation of c-Met in the livers of WT and NK2 transgenic mice was unchanged at each time point after PH. Although we did not understand why only half of NK2 transgenic mice died, there could be differences in circulating levels of NK2 or critical local changes in the transgene expression[25].
Hepatocyte proliferation after PH occurs first in periportal cells, and then in perivenous cells[26]. This indicates that HGF is produced from endothelial and Kupffer cells, and stimulates hepatocyte proliferation via a paracrine mechanism. In addition, HGF acts on hepatocytes located around the central vein by an endocrine mechanism. On the other hand, after CCl4 administration, hepatocyte proliferation occurs randomly in the lobulus[27]. This indicates that HGF is produced by a paracrine mechanism. HGF may therefore have a differential role in hepatocyte proliferation in the PH and CCl4 administration models. That is, NK2 overexpression might be inhibitory to hepatocyte proliferation after PH but not after CCl4-induced acute liver injury.
TNF-α and IL-6 are known to be initiators of liver regeneration after PH[22]. Studies in TNF receptor 1 and IL-6 knock out mice demonstrated the sequence in which TNF-α is induced first after PH, followed by induction of IL-6[22]. Hepatic IL-6 mRNA expression peaked at 4 h after PH in WT mice[28]. TNF receptor 1 knock out mice showed massive lipid accumulation in hepatocytes at 40 h after PH[29] and IL-6 knock out mice showed poor hepatocyte DNA synthesis at 36 h after PH[30]. The observations in these mice are similar to those in NK2 transgenic mice after PH. We have investigated hepatic expression of TNF-α and IL-6 mRNA in NK2 transgenic mice after PH. TNF-α mRNA was increased and IL-6 mRNA was detected in the dying NK2 at 48 h after PH. This result indicates that liver regeneration was impaired in NK2 transgenic mice.
In conclusion, our study demonstrates that over-expression of NK2 prevents liver regeneration after PH.