Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7413
Revised: May 10, 2005
Accepted: May 12, 2005
Published online: December 21, 2005
AIM: To investigate the effect of synbiotics, i.e. probiotics and prebiotics mixture, on the gut microbial ecology and digestive enzyme activities in rats.
METHODS: Forty-eight SD rats weighing about 280 g were used in this study. Rats were divided into three groups according to the contents of probiotics and prebiotics mixture in the feed as control, low and high dose groups. The duration of the experiment was 8 wk.
RESULTS: Compared with the control group, the fecal Lactobacillus and Bifidobacterium counts were significantly increased and the fecal Coliform organism counts were markedly reduced in the low and high dose groups. Concerning the digestive enzyme activity of jejunum, only lactase activity increased in low dose group. However, significant increase of lipase, lactase, sucrase, and isomaltase activities were observed in high dose group.
CONCLUSION: Intake of low and high dosages of probiotics and prebiotics mixture significantly improved the ecosystem of the intestinal tract by increasing the probiotics population and digestive enzyme activities in rats.
- Citation: Yang SC, Chen JY, Shang HF, Cheng TY, Tsou SC, Chen JR. Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol 2005; 11(47): 7413-7417
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7413.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7413
Gastrointestinal microfloras contain hundred different types of microorganisms and are a biologically important component of the body. According to the effect of microbial ecosystem of the human gastrointestinal tract on health, they are divided into two groups: one is probiotics and the other is harmful bacteria. Probiotics, defined as a live microbial food supplement, benefits the host by improving its intestinal microbial balance[1]. Modern perspectives on consumption of probiotics supplements are aimed at consumer well-being, using products enriched with acid bacteria (Lactobacilli), particularly Bifidobacteria, Lactobacillus acidophilus and Lactobacillus bulgaricus, and Streptococcus thermophilus. Through a process of fermentation, the metabolites of these complex microbes produce varying consequences on host health[2,3]. Health claims associated with probiotics supplements include prevention of diarrhea and colitis, antitumorigenic effects, and cholesterol reduction[4-8].
In contrast, prebiotics is a nondigestible nutritional compound (e.g. inulin, oligosaccharide, dietary fiber) that selectively stimulates the growth of endogenous lactic acid bacteria and Bifidobacteria to improve the health of the host[9]. The fermentability and bifidogenic effect of prebiotics have been confirmed with in vitro and in vivo studies[10-13].
The concept of synbiotics has been proposed recently to characterize colonic food with probiotics and prebiotics properties as health enhancing functional food[14]. Research and development of synbiotic products have been increasingly focusing on evidence of functional benefits including resistance to infection, antibacterial activity, and improved immune status[14].
Although there are numerous researches of biotic products focusing on balanced colonic microflora, few reports investigated into the intestinal digestive enzyme activities. Therefore, the role of enteric feeding and the microenvironment in host defense, the effect of synbiotics, i.e. probiotics and prebiotics mixture, on the gut microbial ecology and digestive enzyme activities in rats were investigated in this study.
Forty-eight male SD rats (6 wk old) were purchased from the laboratory animal sources of the National Taiwan University College of Medicine. Rats were fed with a standard laboratory diet and distilled water ad libitum, and housed in an air-conditioned room at 23±2 °C with 12 h of light per day. Experiments were started after the rats reached a weight of about 280 g each and adapted to the individual stainless-steel cages. Rats were randomly divided into three groups of 16 animals: control, low dose and high dose groups. The control group was given 20 g laboratory rodent diet 5001 (PMI Feeds, St. Louis, MO, USA) per day. The low and high dose groups were fed with the synbiotics-containing diets, which contained 20 g/d laboratory rodent diet 5001 and low (1.5 g/kg body weight/day) or high (7.5 g/kg body weight/day) dosages of synbiotics powder (FloraGuard®, Viva Life Science, Costa Mesa, CA, USA). The composition of synbiotics powder is described in Table 1. The duration of the experiment was 8 wk.
| Amount/7.5 g | |
| Thiamin (as thiamin hydrochloride) | 0.375 mg |
| Riboflavin | 0.425 mg |
| Niacin (as niacinamide) | 5 mg |
| Vitamin B6 (as pyridoxine hydrochloride) | 0.5 mg |
| Folate (as folic acid) | 100 μg |
| Vitamin B12 (as cyanocobalamin) | 1.57 μg |
| Biotin | 75 μg |
| Pantothenic acid (as d-calcium pantothenate) | 2.5 mg |
| Inulin from chicory, powdered extract (root) | 250 mg |
| Proprietary blend culture count | 10 billion2 |
| Lactobacillus acidophilus and Lactobacillus bulgaricus | 2.7 billion |
| Bifidobacterium bifidum and Bifidobacterium longum | 6.7 billion |
| Streptococcus thermophilus | 0.6 billion |
Before the rats were killed, feces were collected from each rat on the final 7 d of the experimental period. The feces were freeze-dried and weighed. The wet weight, dry weight, moisture content, and physical appearance of the feces were recorded.
At 10:00 a.m., the day before the end of the experiment, all rats were anesthetized by ethyl ether inhalation and fecal samples were collected in sterile centrifuged tubes containing 9 mL of anaerobic dilution buffer (0.2% gelatin, 0.05% cysteine, and 0.0002% resazurin). Samples were brought to the laboratory within 2 h after defecation. Additionally, each sample was duplicated and inoculated onto the agar by the spread plate method for plate count determination. Ten-fold serial dilutions of the fecal samples (10-1-10-6) were m-ade in sterile physiological saline (9 g/L) and 50 μL was inoculated onto the following agar plates.
CDC anaerobe blood agar plates (Oxoid CM271, Basingstoke, Hants., UK) was used for the detection of total aerobic bacterial flora. Blood agar plates were incubated aerobically at 37 °C for 24 h.
Detection of LactobacillusLactobacillus anaerobic MRS with vancomycin and bromocresol green (LAMVAB) was used for the detection of lactobacilli. LAMVAB (pH 5) was prepared with MRS broth (104.4 g/L), cysteine-HCl (0.5 g/L), bromocresol green (0.05 g/L), agar (40 g/L) and vancomycin hydrochloride (>95% purity, 2 mg/mL) according to the method described previously[15]. LAMVAB agar plates were pre-reduced in the anaerobic cabinet for 24 h before the inoculation and were incubated in the anaerobic cabinet at 37 °C for 48 h after inoculation.
Plate count of viable Bifidobacteria Modified Bifidobacterium iodoacetate medium-25 (BIM-25) was used for the enumeration of bifidobacteria. All plates were incubated for 72 h at 37 °C.
Detection of Coliform organisms Detection of Coliform organisms in fecal samples collected from rats were directly inoculated onto the Endo agar plates and incubated anaerobically in McIntosh Fildes jar at 37 °C for 48 h. Fourteen grams of dehydrated Endo agar media was dissolved in 500 mL of water and brought to a rolling boil. The media were then autoclaved at 121 °C for 15 min. Twenty milliliters of the agar was poured into the Petri plates that were sterilized under UV light and allowed to cool. The Endo agar plates were capped and stored upside down in the refrigerator for future use.
Preparation of tissues After 8 wk, all rats were anesthetized by ethyl ether inhalation and killed. Blood was collected via the abdominal aorta. The small intestine was immediately removed, and then washed in ice-cold physiological saline (9 g/L NaCl). The length of the small intestine was measured. The mucosal cells of the jejunums (about 10 cm), where sucrase activity is most highly concentrated, were scraped off with a piece of glass and homogenized in ice-cold distilled water. The homogenates were centrifuged at 7 000×g for 10 min at 4 °C. Supernatants were transferred into new Eppendorf tubes and stored at -80 °C for further analysis.
Measurement of lipase and disaccharidase activities Lipase activity was measured with a commercial kit (lipase assay kit, LI-186, Randox Laboratories Ltd, Co.).
Sucrase, isomaltase and lactase activities from mucosal extracts were measured according to the modified method of Dahlqvist with some modifications[16]. Substrate buffer solution containing maltose, sucrose, or lactose was incubated with a diluted sample for 60 min at 37 °C. The reaction was terminated by adding O-dianisidine, and released glucose was determined by means of a Tris glucose-oxidase (TGO) procedure (Tris-HCl, 100 mL, 0.5 mol/L, pH 7.0; glucose oxidase, 1.7 mg; peroxidase, 0.5 mg and Triton X-100, 1 mL) and the samples were analyzed by spectrophotometry at 420 nm. One unit of disaccharidase activity was defined as the amount of the enzyme that can hydrolyze 1 μmol/L of substrate equivalent per minute under the assay conditions. The specific disaccharidase activities were expressed as units/g protein.
Total protein concentrations Total protein concentrations of samples were spectrophotometrically estimated by the method of Biuret with bovine serum albumin as a standard.
All data were expressed as the mean±SD. One-way analysis of variance and Fisher’s least significant difference test were used to compare the differences of means using the SAS software (version 8.2, SAS Institute, Cary, NC, USA). Statistical significance was assigned at the 0.05 level.
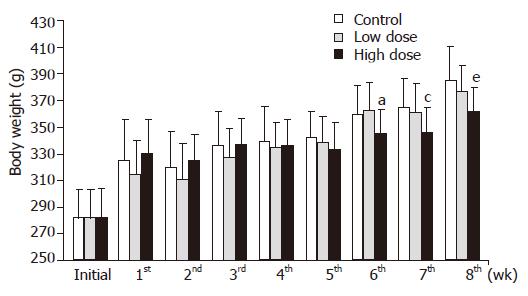
There was no difference in the feed intake in each group (Table 2). Body weight changes are shown in Figure 1. There were no changes in body weight among the three groups until 5 wk. However, the rats of high dose group showed significantly decreased body weights, as compared with the rats of control or low dose groups at the 6th, 7th and 8th wk.
| Wk | Control (g) | Low dose (g) | High dose (g) |
| 1 | 19.9±0.6 | 19.4±1.7 | 19.8±0.7 |
| 2 | 20.0±0.1 | 19.6±1.4 | 19.9±0.2 |
| 3 | 19.9±0.4 | 19.9±0.2 | 19.9±0.2 |
| 4 | 18.5±1.5 | 18.1±0.1 | 18.9±0.2 |
| 5 | 19.9±0.3 | 19.9±0.3 | 20.0±0.4 |
| 6 | 19.9±0.1 | 19.8±0.4 | 19.8±0.5 |
| 7 | 20.0±0.1 | 20.0±0.1 | 19.8±0.8 |
| 8 | 19.9±0.3 | 20.0±0.3 | 19.4±0.3 |
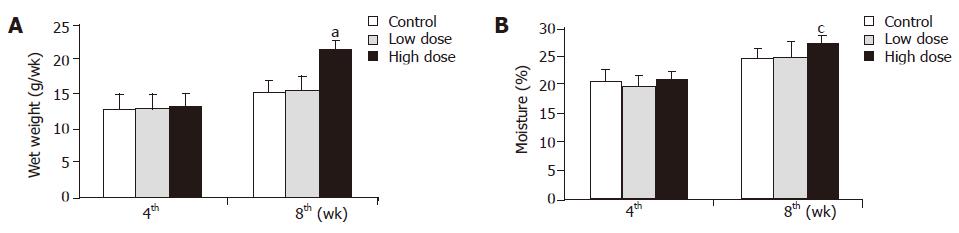
The fecal weight and moisture in the low dose group did not differ at the 4th and 8th wk, in contrast to those of the control group (Figure 2). Although, the fecal weight and moisture in high dose group did not change at the 4th wk, they significantly increased at the 8th wk (Figure 2).
Table 3 presents the intestinal microorganisms in each group. There was no change in anaerobic bacteria counts in each group. In addition, the Lactobacillus and Bifidobacteria counts were significantly increased in low and high dose groups as compared with the control group. On the contrary, the count of Coliform organisms was significantly decreased in low and high groups.
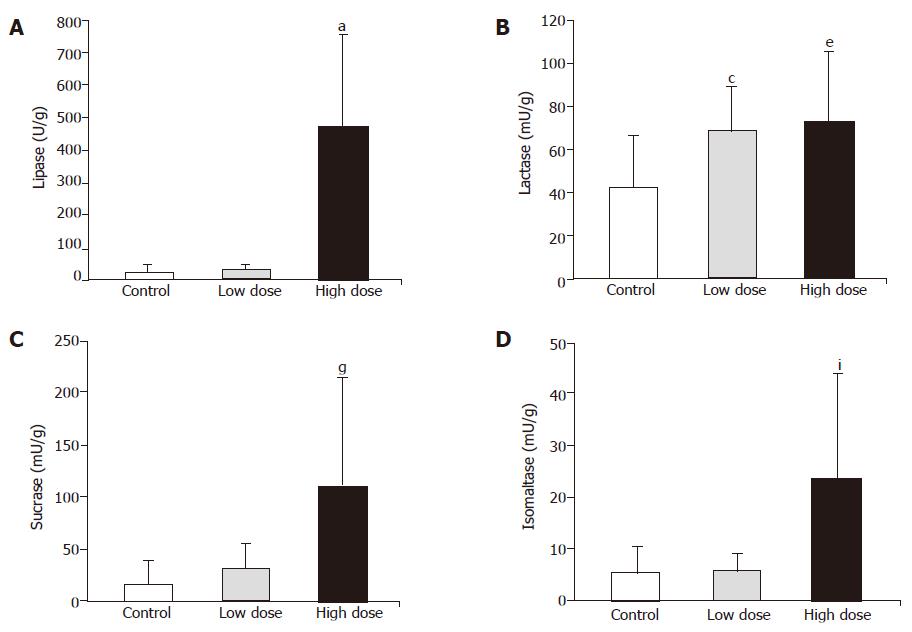
As shown in Figure 3, lipase, sucrase and isomaltase activities did not change, but lactase was significantly higher in low dose group, than in control group. On the other hand, not only lipase but also disaccharidase activities significantly increased in high dose group as compared with control group (Figure 3).
In this study, the effect of synbiotics containing probiotics and prebiotics on gut microbial ecology and digestive enzyme activities were investigated.
Many researches proved that consumption of prebiotics, such as inulin, could stimulate intestinal peristalsis by means of increasing fecal bulk and moisture[9,17]. The mechanism of action has been observed that indigestible water-soluble dietary fiber may incorporate a lot of water in the intestine[9]. Inulin consists of 2-60 fructose units linked by a β-(2→1)-glycosidic linkage often with a terminal glucose unit[9]. These fructans are not hydrolyzed by the digestive enzymes in the small intestine; they reach the colon unabsorbed and are utilized selectively as a substrate for the growth of bifidobacteria. The most widely accepted effect of inulin is to enlarge both the number and the proportion of fecal bifidobacteria[18]. These bacteria are recognized for creating conditions unfavorable for the growth of potentially pathogenic organisms, such as Coliform organism[19,20]. However, there seems to be no increase in the total bacteria number or a change in the anaerobe number[21].
In this study, rats that ingested high dose synbiotics showed lower body weight after 6 wk and higher fecal weight after 8 wk (Figures 1 and 2). Thus, it was speculated that consumption of synbiotics containing inulin may reduce body weight by means of improving the fecal bulk and moisture. Furthermore, the intestinal side-effect of synbiotics powder consumption, including diarrhea or constipation, was not observed in rats during the experimental period. This result may support the clinical application of synbiotics to weight management. In addition, it was also observed that the Lactobacillus and Bifidobacteria counts were significantly increased, but there was no change in the anaerobic bacteria counts in rats fed with low and high dose synbiotics, while the count of Coliform organisms, the harmful bacteria, were significantly reduced in rats fed with low and high dose synbiotics in this study (Table 3). These results were similar to our previous researches. There are two possibilities to explain the results. The first is the fermentation of inulin that provided short-chain fatty acids, stimulating the proliferation of Lactobacillus and Bifidobacteria and suppressing potential pathogenic organisms in the gut; and the second was the supplement of probiotics, including Bifidobacteria, Lactobacillus acidophilus, that directly improved the total number of these bacteria.
Some reported advantages of bifidobacterial proli-feration in the human gut included the production of digestive enzymes. However, these reports were limited in in vitro testing[2]. Jiang et al[22]. proved that Bifidobacterium longum improved the lactose intolerance in human beings by secreting the lactase to the gut. In this study, jejunal lactase activity was improved in both low dose and high dose intake of synbiotics, and furthermore, lipase, sucrase and isomaltase activities were also increased in rats fed with high dose synbiotics (Figure 3). The causes for the improvement of digestive enzyme activity possibly are that synbiotics created the healthy gastrointestinal microbial ecology or modified the secretion of bacterial enzyme. Delzenne et al[23]. also suggested that polyamines may be synthesized from dietary fermentable substrates such as inulin or guar gum by bacteria. The exogenous sources of polyamines seemed to be essential for small intestinal and colonic mucosal growth and development[23]. Thus, the high digestive enzyme activities in rats fed with synbiotics powder were possibly caused by the well growth and high turnover rate of intestinal mucosa.
In conclusion, intake of low and high dose of synbiotics, which was the combination of probiotics and prebiotics, significantly improved the ecosystem of intestinal tract by increasing the probiotics population and jejunal digestive enzyme activities in rats. Based on this study, another possibility in the benefits of synbiotics is the weight management, and the clinical trial is still necessary in future.
Science Editor Ma JY and Guo SY Language Editor Elsevier HK
| 1. | Djouzi Z, Andrieux C, Degivry MC, Bouley C, Szylit O. The association of yogurt starters with Lactobacillus casei DN 114.001 in fermented milk alters the composition and metabolism of intestinal microflora in germ-free rats and in human flora-associated rats. J Nutr. 1997;127:2260-2266. [PubMed] |
| 2. | Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052S-1057S. [PubMed] |
| 3. | Cui HH, Chen CL, Wang JD, Yang YJ, Sun Y, Wang YD, Lai ZS. The effects of bifidobacterium on the intestinal mucosa of the patients with ulcerative colitis. Zhonghua NeiKe ZaZhi. 2003;42:554-557. [PubMed] |
| 4. | Duffy LC, Zielezny MA, Riepenhoff-Talty M, Dryja D, Sayahtaheri-Altaie S, Griffiths E, Ruffin D, Barrett H, Rossman J, Ogra PL. Effectiveness of Bifidobacterium bifidum in mediating the clinical course of murine rotavirus diarrhea. Pediatr Res. 1994;35:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Yolken RH, Ojeh C, Khatri IA, Sajjan U, Forstner JF. Intestinal mucins inhibit rotavirus replication in an oligosaccharide-dependent manner. J Infect Dis. 1994;169:1002-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Favier C, Neut C, Mizon C, Cortot A, Colombel JF, Mizon J. Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn's disease. Dig Dis Sci. 1997;42:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73:451S-455S. [PubMed] |
| 8. | Tahri K, Crociani J, Ballongue J, Schneider F. Effects of three strains of bifidobacteria on cholesterol. Lett Appl Microbiol. 1995;21:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1062] [Cited by in RCA: 897] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Wang X, Gibson GR. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J Appl Bacteriol. 1993;75:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 404] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Fuller R, Gibson GR. Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol Suppl. 1997;222:28-31. [PubMed] |
| 12. | Gallaher DD, Stallings WH, Blessing LL, Busta FF, Brady LJ. Probiotics, cecal microflora, and aberrant crypts in the rat colon. J Nutr. 1996;126:1362-1371. [PubMed] |
| 13. | Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127:130-136. [PubMed] |
| 14. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] |
| 15. | Hartemink R, Rombouts FM. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. J Microbiol Methods. 1999;36:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Dahlqvist A. Assay of intestinal disaccharidases. Enzymol Biol Clin (Basel). 1970;11:52-66. [PubMed] |
| 17. | Bouhnik Y, Flourié B, Riottot M, Bisetti N, Gailing MF, Guibert A, Bornet F, Rambaud JC. Effects of fructo-oligosaccharides ingestion on fecal bifidobacteria and selected metabolic indexes of colon carcinogenesis in healthy humans. Nutr Cancer. 1996;26:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 125] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Roberfroid MB, Van Loo JA, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128:11-19. [PubMed] |
| 19. | Gibson GR, Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994;77:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 394] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Roberfroid MB. Health benefits of non-digestible oligosaccharides. Adv Exp Med Biol. 1997;427:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71:1682S-1687S; discussion 1682S-1687S;. [PubMed] |
| 22. | Jiang T, Mustapha A, Savaiano DA. Improvement of lactose digestion in humans by ingestion of unfermented milk containing Bifidobacterium longum. J Dairy Sci. 1996;79:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |