Published online Dec 21, 2005. doi: 10.3748/wjg.v11.i47.7391
Revised: March 3, 2005
Accepted: March 10, 2005
Published online: December 21, 2005
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. The major etiologies and risk factors for the development of HCC are well defined and some of the multiple steps involved in hepatocarcinogenesis have been elucidated in recent years. Despite these scientific advances and the implementation of measures for the early detection of HCC in patients at risk, patient survival has not improved during the last three decades. This is due to the advanced stage of the disease at the time of clinical presentation and limited therapeutic options. The therapeutic options fall into five main categories: surgical interventions including tumor resection and liver transplantation, percutaneous interventions including ethanol injection and radiofrequency thermal ablation, transarterial interventions including embolization and chemoembolization, radiation therapy and drugs as well as gene and immune therapies. These therapeutic strategies have been evaluated in part in randomized controlled clinical trials that are the basis for therapeutic recommendations. Though surgery, percutaneous and transarterial interventions are effective in patients with limited disease (1-3 lesions, <5 cm in diameter) and compensated underlying liver disease (cirrhosis Child A), at the time of diagnosis more than 80% patients present with multicentric HCC and advanced liver disease or comorbidities that restrict the therapeutic measures to best supportive care. In order to reduce the morbidity and mortality of HCC, early diagnosis and the development of novel systemic therapies for advanced disease, including drugs, gene and immune therapies as well as primary HCC prevention are of paramount importance. Furthermore, secondary HCC prevention after successful therapeutic interventions needs to be improved in order to make an impact on the survival of patients with HCC. New technologies, including gene expression profiling and proteomic analyses, should allow to further elucidate the molecular events underlying HCC development and to identify novel diagnostic markers as well as therapeutic and preventive targets.
- Citation: Blum HE. Hepatocellular carcinoma: Therapy and prevention. World J Gastroenterol 2005; 11(47): 7391-7400
- URL: https://www.wjgnet.com/1007-9327/full/v11/i47/7391.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i47.7391
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide[1-10]. The incidence ranges from <10 cases per 100 000 persons in North America and Western Europe to 50-150 cases per 100 000 persons in parts of Africa and Asia, where HCC is responsible for a large number of cancer deaths. However, a rise in the incidence and mortality of HCC, most likely reflecting the increased prevalence of hepatitis C virus (HCV) infection, has recently been observed in most industrialized countries[11-14].
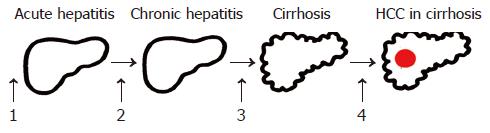
The major etiologies of HCC are well defined (Table 1). An elevated body mass index, especially in men[15], and diabetes mellitus[9] are included among the well-known factors. Some of the steps involved in the molecular pathogenesis of HCC have been elucidated in the recent years. As for most types of cancer, hepatocarcinogenesis is a multistep process involving different genetic alterations that ultimately lead to the malignant transformation of hepatocytes. While significant progress has been made in recognizing the sequence of events involved in other forms of cancer, most notably in colorectal cancer and certain hematopoietic malignancies, the molecular contribution of the multiple factors and their interactions in hepatocarcinogenesis are still poorly understood. HCCs are phenotypically (morphology and microscopy) and genetically heterogenous tumors, possibly reflecting in part the heteroge neity of etiologic factors implicated in HCC development, the complexity of hepatocyte functions and the late stage at which HCCs usually become clinically symptomatic and detectable. Malignant transformation of hepatocytes may occur regardless of the etiologic agent through a pathway of increased liver cell turnover, induced by chronic liver injury and regeneration in a context of inflammation, immune response, and oxidative DNA damage. This may result in genetic alterations, such as activation of cellular oncogenes, inactivation of tumor suppressor genes, possibly in cooperation with genomic instability, including DNA mismatch repair defects and impaired chromosomal segregation, overexpression of growth and angiogenic factors, and telomerase activation[16-23]. Chronic viral hepatitis B, C, and D, alcohol, metabolic liver diseases such as hemochromatosis and α-1-antitrypsin deficiency as well as non-alcoholic fatty liver disease may act predominantly through this pathway of chronic liver injury, regeneration and cirrhosis. Accordingly, the major clinical risk factor for the development of HCC is liver cirrhosis since 70-90% of HCCs develop into a cirrhotic liver. Most HCCs occur after many years of chronic hepatitis that provides the mitogenic and mutagenic environments to precipitate random genetic alterations resulting in the malignant transformation of hepatocytes and HCC development.
| Chronic viral hepatitis B, C, D |
| Toxins (e.g., alcohol, aflatoxins) |
| Hereditary metabolic liver diseases (e.g., hereditary hemochromatosis, |
| α-1-antitrypsin deficiency) |
| Autoimmune hepatitis |
| Overweight, especially in males, and diabetes mellitus; nonalcoholic |
| steatohepatitis (NASH) or nonalcoholic fatty liver disease (NAFLD) |
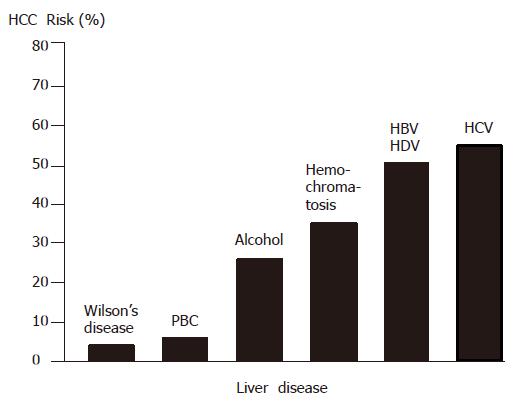
The HCC risk in patients with liver cirrhosis depends on the activity, duration and etiology of the underlying liver disease (Figure 1). Clinical and biological variables (age, anti-HCV positivity, PTT and platelet count) allow to further identify a subset of cirrhotic patients with the highest risk of HCC development[24]. Coexistence of etiologies, such as hepatitis B virus (HBV) and HCV infection, HBV infection and aflatoxin B1[23,25], HBV/HCV infection and alcohol or diabetes mellitus[26], or HCV infection and liver steatosis[27], increases the relative risk of HCC development. Also, occult HBV infection (anti-HBc positive only) carries a significant HCC risk[28,29]. In general, HCCs are more frequent in males than in females and the incidence increases with age. On the other hand, there is evidence that HBV and possibly HCV under certain circumstances play an additional direct role in the molecular pathogenesis of HCC. Finally, aflatoxins can induce mutations of the p53 tumor suppressor gene, thus pointing to the contribution of an environmental factor to tumor development at the molecular level. Furthermore, in a transgenic mouse model it has been shown that chronic immune-mediated liver cell injury without environmental or infectious agents is sufficient to cause HCC[30] and that inhibition of cytotoxic T lymphocyte-induced apoptosis and chronic inflammation by neutralization of the Fas ligand and prevents HCC development in this model[31]. In addition, in a transgenic mouse model it has been demonstrated that NF-κB may be the link between inflammation and HCC development[32,33]. Finally, individual polymorphisms of drug-metabolizing enzymes, such as cytochrome P450 oxidases, N-acetyltransferases and glutathione-S-transferase, may contribute to the genetic susceptibility to HCC development[34].
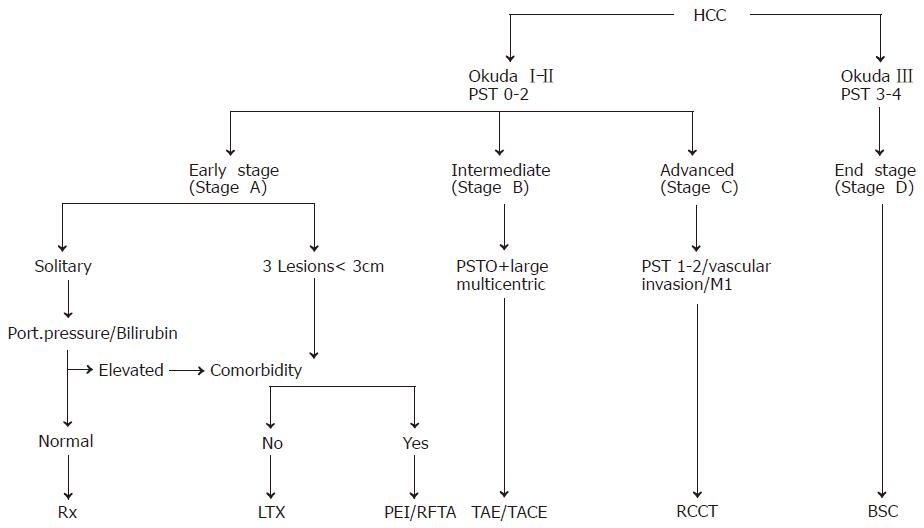
For the staging of HCCs, five systems have been proposed for the assessment of the extent and prognosis of the disease[35,36]: the Okuda staging system (Table 2)[37], the TNM classification and its modification by the Union International Contre Cancer (UICC), the Barcelona Clinic Liver Cancer (BCLC) classification[38] and the Cancer of the Liver Italian Program (CLIP) score[39]. The Okuda staging system is very effective for the identification of a subgroup of patients (Okuda III) with a very good prognosis of patients who should be treated with best supportive care (BSC) only. The BCLC classification appears especially useful for the selection of treatment but has not been independently validated. The CLIP score is superior to the Okuda staging system but has not been systematically assessed in patients undergoing resection or liver transplantation. Thus, one staging system is not clearly superior to the others. The natural course of the disease and the median survival of patients with HCC depend on the stage of the disease at the time of diagnosis. In patients with CLIP score 0 or Okuda stage I, the median survival is in the range of 23-69 mo, while in patients with CLIP score 3-5 or Okuda stage III, the median survival is only 1-14 mo[4]. The staging system is clinically most important for the appropriate choice of therapeutic strategy for individual patients.
| Tumor mass | <50% of liver | ≥50% of liver |
| Ascites | No | Yes |
| Albumin (g/L) | >3 | ≤3 |
| Bilirubin (mg/dL) | <3 | ≥3 |
| Points | 0 | 1 |
| Stage I | 0 points | |
| Stage II | 1-2 points | |
| Stage III | 3-4 points |
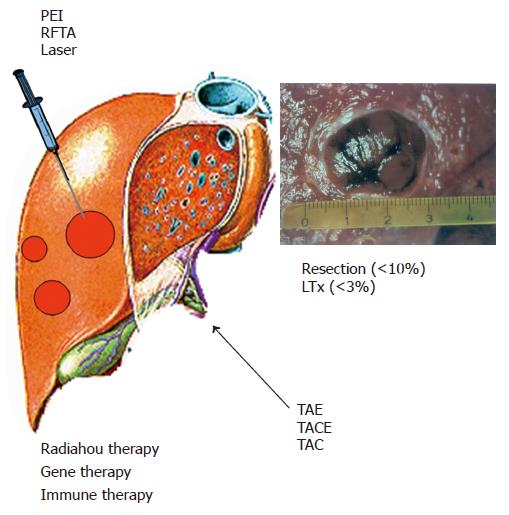
Therapies for HCC can be divided into four categories: surgical interventions (tumor resection and liver transplantation), percutaneous interventions (ethanol injection, radiofrequency thermal ablation), transarterial interventions (embolization, chemoperfusion, or chemoembolization) and drugs including gene and immune therapy (Figure 2). Potentially curative therapies are tumor resection, liver transplantation, and percutaneous interventions that can result in complete responses and improved survival in a large number of patients. In selected cases, transarterial interventions result in palliation with good response rates and improved survival in some cases. Drugs as well as conventional radiotherapy have no proven efficacy.
Till date, surgical, percutaneous and transarterial interventions have not been compared in randomized controlled trials. Tumor resection and transplantation can achieve a 5-year survival rate of 60-70% in selected patients transplantation is the best treatment for patients with single lesions and advanced liver diseases, such as decompensated cirrhosis and multicentric small tumors. Percutaneous interventions, again in selected patients, result in a 5-year survival rate of 40-50%. The following different therapeutic options as well as primary and secondary HCC prevention are discussed in detail.
Resection in patients without concomitant liver cirrhosis (5% in Western countries, 40% in Sub-Sahara Africa and Asia), HCC resection is the treatment of choice with a low rate of life-threatening complications. By comparison, in the majority of patients with cirrhosis, strict selection is required to avoid resection-related complications, especially postoperative liver failure[40]. Apart from bilirubin and albumin concentration as well as platelet count and indocyanine green clearance[14,42], a recent study has identified an elevated serum concentration of 7s-collagen as an independent risk factor for postoperative liver failure[43].
Resection-related mortality should be <1-3%, and the 5-year survival rate should be >50%. In patients with normal liver function (normal indocyanine green retention rate and bilirubin level), absence of clinically relevant portal hypertension (hepatic venous pressure gradient <1.33 kpa, no esophageal varices, no splenomegaly, platelet count >100×109/L) and one asymptomatic HCC lesion only, the 5-year survival rate of 70% can be achieved. By comparison in patients with clinically relevant portal hypertension, the 5-year survival rate is about 50% only and in patients with portal hypertension and evidence of impaired liver function, the 5-year survival rate is even lower.
After successful HCC resection, tumor recurrence in the cirrhotic liver (local recurrence as well as de novo tumors) in about 70% of patients after 5 years is a major clinical problem. The risk of recurrence is especially high in patients with microvascular invasion and/or additional tumor nodules[41,44]. Therefore, strategies aimed at secondary HCC prevention are of paramount importance.
Liver transplantation Liver transplantation is in principle the optimal therapeutic option for HCCs, because it simultaneously removes the tumor and the underlying cirrhosis, including the risk of HCC recurrence[40,45-47]. While broad selection criteria applied previously led to poor results with the recurrence rate of about 50% and the 5-year survival rate <40%, the current criteria for liver transplantation in patients with HCC (1 lesion <5 cm in diameter or maximum 3 lesions <3 cm in diameter) result in the 5-year survival rate of 70% or more and the recurrence rate <15%[48-50]. Possibly these criteria can be extended in future, depending on more experiences based on the stage of the disease, macrovascular invasion, histopathological characteristics (histopathology, aneuploidy, microvascular invasion) as well as DNA and RNA chip data (molecular signature, proteomic signature and others)[51,52].
Clinically, it is most important to shorten the waiting time for transplantation to <6 mo. This is difficult to achieve with cadaveric liver transplantation due to the shortage of donors. With a waiting time of >12 mo in some Western countries, the drop-out rate of patients is 20-50%. To bridge the time to transplantation and to prevent tumor progression, neoadjuvant treatments, such as percutaneous and transarterial interventions may lead to an improved outcome[53]. While marginal livers, domino donors, and split liver transplantation have no major impact, living donor liver transplantation has been shown to be an alternative to cadaveric liver transplantation. Around 3 000 interventions have been done worldwide. However, living donor liver transplantation is a complex procedure with a morbidity of 20-40% and a donor mortality of 0.3-0.5%[50,54,55]. Therefore, a very careful selection of patients and donors, including consideration of ethical, societal and legal issues is central to the successful implementation of living donor liver transplantation for the treatment of HCC patients[56].
Percutaneous intervention is the best option for small unresectable HCCs[57-59]. Tumor ablation can be achieved chemically by percutaneous ethanol injection (PEI) or acetic acid injection (PAI) or thermally by radiofrequency thermal ablation (RFTA), microwave heat-induced thermotherapy (HiTT), laser-induced thermotherapy (LiTT), or cryoablation. Apart from percutaneous interventions, these techniques can be applied also laparoscopically or after laparotomy.
Percutaneous ethanol injection PEI is the most widely used technique[60,61]. It is safe, easy to perform, inexpensive and can achieve complete tumor response rate of 90-100% in HCCs smaller than 2 cm in diameter, 70% in HCCs (3 cm in diameter) and 50% in HCCs (5 cm in diameter). Patients with liver cirrhosis Child A with complete responses can achieve a 5-year survival rate of 50% or more. Therefore, PEI is the procedure of choice for patients with a single HCC lesion smaller than 5 cm in diameter or with up to three lesions smaller than 3 cm in diameter (Figure 3).
Radiofrequency thermal ablation RFTA is an alternative to PEI[58,59,62,63]. Several devices are available that can be applied percutaneously, laparoscopically, or during laparotomy. The efficacy of RFTA is similar to that of PEI but requires generally only a single session. While being more expensive than PEI, RFTA offers a better local tumor control and a potential advantage of allowing the ablation of tumors larger than 5 cm in diameter especially when newer generation devices are used. However, the 5-year survival rate after complete response to RFTA is currently similar to that of PEI (around 30-40%) depending on the child stage of the underlying liver cirrhosis. In a review of 3 670 patients treated with RFTA, the mortality is 0-5% and the complication rate is 8-9%[64]. Predictors of treatment response are tumor size and morphology (well encapsulated vs invasive).
Percutaneous HCC ablation by PEI and/or RFTA when considered together is an effective treatment for patients with HCCs that prolongs the tumor-free and overall survival time, especially if surgery is not feasible. This strategy has been evaluated also for the treatment of liver metastases[65].
Transarterial embolization and chemoembolization are the most widely used treatments for HCCs which are unresectable or cannot be effectively treated with percutaneous interventions[66-69]. Embolization agents may be administered alone (embolization) or after selective intra-arterial chemotherapy (generally doxorubicin, mitomycin or cisplatin) or in combination with lipiodol (chemoembolization). Transarterial embolization or chemoembolization results in partial responses in 15-55% of patients, delays tumor progression and vascular invasion, and prolongs the survival time compared to conservative management. The most important aspect is the selection of patients, i.e., patients should have preserved liver function (Child A) and asymptomatic multinodular tumors without vascular invasion or extrahepatic spread[67,69]. In patients with advanced liver disease (Child B or C), treatment-induced liver failure may offset the antitumor effect or survival benefit of the intervention. As has been shown recently, postoperative adjuvant TACE may improve survival in patients with risk factors for residual tumor[70].
While radiotherapy plays only a minor role in the treatment of primary HCC, selective intra-arterial injection of 131iodine-labeled lipiodol has been performed in some patients[71] but needs further clinical evaluation before a recommendation can be made. Furthermore, high dose proton beam radiotherapy and external beam radiation as well as Yttrium-90 microsphere treatment have been recently explored in clinical trials in patients with unresectable HCC[72-74]. These strategies will certainly be further explored in clinical studies and may become a treatment option in future.
A number of chemotherapeutic, hormonal and other drugs (Table 3) have been evaluated in clinical trials[75-77]. While most chemotherapeutic agents, such as tamoxifen[78], octreotide[79] and interferon[66], have not been shown to be effective in randomized controlled clinical trials, there are a number of substances that may deserve further clinical evaluation, such as gemcitabine[80,81], thymostimulin[82], α-1-thymosin[83], pravastatin[84], thalidomide[85] and megestrol acetate[86], antiangiogenic small molecules, Cox-2 inhibitors in combination with capecitabine and possibly others. Till date, however, none of these drugs can be recommended outside clinical studies.
| 5-Fluorouracil |
| Capecitabine |
| Doxorubicin |
| Epirubicin |
| Etoposide |
| Cisplatin |
| Gemcitabine |
| Mitoxantrone |
| Interferon alpha |
| Megestrol acetate |
| Tamoxifen |
| Octreotide |
| Thalidomide |
| Thymophysin |
| α-1-thymosin |
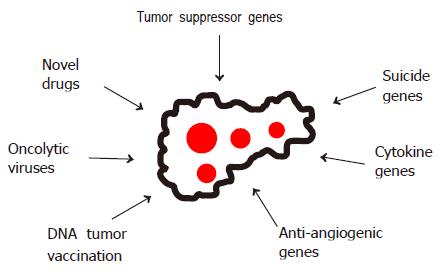
In view of the limited therapeutic options for advanced HCCs, a number of experimental strategies are being evaluated (Figure 4), including gene and immune therapies based on suicide, cytokine and antiangiogenic genes or DNA vaccination with tumor-specific genes[87-90], oncolytic viruses[91] as well as novel drugs such as 3-bromopyruvate[92].
HCC prevention falls into two categories. Primary prevention that is aimed at the prevention of HCC development in patients with chronic liver diseases of different etiologies and secondary prevention that is aimed at preventing the recurrence and/or the development of new HCC lesions after successful surgical or non-surgical HCC treatment[93,94].
Primary HCC prevention Primary prevention is aimed at the interference with HCC development at four stages (Figure 5).
Stage 1: Interventions at this step are aimed at the prevention of acquired liver diseases. Apart from avoiding liver toxins, including alcohol and certain drugs, or infections with HBV or HCV by hygienic measures, avoiding parenteral exposure to blood, blood products or contaminated needles etc., a prime example is vaccination against HBV infection using commercially available active and passive vaccines. Several HBV vaccines using natural or recombinant hepatitis B surface antigen (HBsAg) from different sources are well introduced in clinical practice and universal vaccination in Taiwan has indeed already resulted in a decline of the incidence of HCCs[95]. In addition, novel HBV vaccination strategies are being explored, including a novel triple HBsAg recombinant vaccine[96], epidermal HBsAg powder immunization[97] as well as oral immunization using HBsAg transgenic plants[98-100]. Furthermore, DNA vaccination has been shown in animal models to induce antibodies against HBsAg/anti-HBs[101,102] even after topical application to the skin. For the prevention of HCV infection, however no effective vaccine is available till date. While several HCV vaccination concepts are being evaluated, including HCV proteins[103], HCV-like particles[104] as well as intravenous, intrahepatic, intraepidermal, intramuscular or oral cDNA immunization[105-109], it is not expected that a vaccine against HCV infection will become commercially available within the next few years.
Stage 2: Interventions at this step are aimed at the early treatment of acute liver diseases, thereby blocking their transition into chronic hepatitis that carries the risk of developing liver cirrhosis and its sequelae, including HCC development. While the principles mentioned above regarding liver toxins can also be applied here, the early diagnosis and treatment of inherited liver diseases, such as Wilson’s disease and hemochromatosis, are of paramount importance. Furthermore, recent studies suggest that early treatment of acute HCV infection prevents its progression to chronic hepatitis C[110-112].
Stage 3: Interventions at this step are aimed at the prevention of the progression of chronic hepatitis to liver cirrhosis that carries a high risk of HCC development. Apart from avoiding liver toxins and long-term use of high dose androgens or other anabolic steroids, the treatment of chronic hepatitis is of paramount importance. This includes the treatment of inherited, cholestatic or autoimmune liver diseases as well as the treatment of chronic viral hepatitis B or C. Reduction of iron overload by phlebotomy, for example, has been shown to stop the progression of hemochromatosis to liver cirrhosis and HCC. Treatment of chronic hepatitis B with interferon alpha or nucleoside analogs[113-118] and chronic hepatitis C with interferon alpha and now the combination of interferon alpha and ribavirin has demonstrated biochemical, virological, and histopathological improvements[119-123] and a lower incidence of HCC development[124-126].
Stage 4: Interventions at this step are aimed at interfering with the molecular events leading to HCC development, usually in a cirrhotic liver. These strategies include all treatment modalities detailed above (stage 3) as far as they can be implemented in patients with compensated or decompensated liver cirrhosis. In addition, some of the measures to prevent HCC recurrence after successful HCC treatment (secondary prevention) should in principle be useful for HCC prevention at this stage of the disease. Furthermore, some concepts of molecular therapy of HCCs should be applicable also in the prevention of HCCs. Without experimental pre-clinical data on these issues, it would be premature to discuss their potential clinical impact.
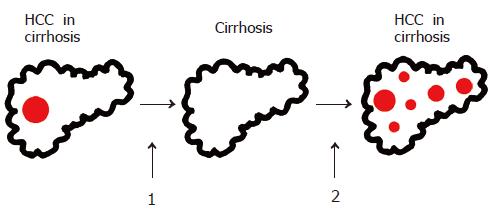
Secondary HCC prevention The prevention of a local recurrence and/or the development of new HCC lesions in patients after successful surgical or non-surgical HCC treatment (Figure 6) is of paramount importance and can significantly improve disease-free and overall patient survival.
After successful HCC resection or non-surgical ablation, HCC recurrence in the remaining cirrhotic liver is the major limitation of life expectancy of these patients. The probability of recurrence is about 50% within 3 years after successful treatment[38,127]. Strategies to prevent HCC recurrence are therefore central to the improvement of survival of HCC patients after initial cure. Apart from liver transplantation after successful resection[44], the strategies explored to date include administration of polyprenoic acid (an acyclic retinoid)[128] interferon alpha[129] and interferon beta[130]. Furthermore, adoptive immunotherapy[131] and intra-arterial injection of 131iodine-labeled lipiodol[132,133] have been evaluated in clinical studies. All these interventions can result in lower HCC recurrence rates. These findings have to be confirmed in larger randomized controlled studies, demonstrating that a clear clinical benefit before secondary prevention with one of the strategies mentioned above should enter clinical practice.
HCC is one of the most common malignant tumors in some areas of the world with an extremely poor prognosis HCC treatment is based on randomized controlled trials and many observational studies. Treatment options fall into four main categories: surgical interventions including tumor resection and liver transplantation, percutaneous interventions including ethanol injection and RFTA, transarterial interventions including embolization and chemoembolization, and drugs as well as gene and immune therapies. Though surgery as well as percutaneous and transarterial interventions are effective in patients with limited disease (up to three lesions smaller than 3 cm in diameter or one lesion smaller than 5 cm in diameter) and compensated underlying liver disease (cirrhosis Child A), at the time of diagnosis more than 80% patients present with multicentric HCC and advanced liver disease or comorbidities that restrict the therapeutic measures to BSC.
In order to reduce the morbidity and mortality of HCC, early diagnosis and the development of novel systemic therapies for advanced disease, including drugs, gene and immune therapies as well as primary HCC prevention are of paramount importance. Furthermore, secondary HCC prevention after successful therapeutic interventions needs to be improved in order to achieve better survival of patients with HCC. New technologies, including gene expression profiling and proteomic analyses, should allow to further elucidate the molecular events underlying HCC development and to identify novel diagnostic markers as well as therapeutic and preventive targets.
| 1. | Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 398] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430 DOI : 10.1016/S0168-8278(01)00130-1. |
| 3. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 857] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 4. | Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 419] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3294] [Article Influence: 143.2] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 398] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 8. | Kiyosawa K, Umemura T, Ichijo T, Matsumoto A, Yoshizawa K, Gad A, Tanaka E. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 729] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 10. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1832] [Article Influence: 83.3] [Reference Citation Analysis (3)] |
| 11. | Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-1994. Lancet. 1997;350:1142-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 223] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 13. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 14. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 685] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 15. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5430] [Cited by in RCA: 5355] [Article Influence: 232.8] [Reference Citation Analysis (2)] |
| 16. | Ozturk M. Genetic aspects of hepatocellular carcinogenesis. Semin Liver Dis. 1999;19:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Bergsland EK. Molecular mechanisms underlying the development of hepatocellular carcinoma. Semin Oncol. 2001;28:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1103] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 19. | Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 375] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 20. | Suriawinata A, Xu R. An update on the molecular genetics of hepatocellular carcinoma. Semin Liver Dis. 2004;24:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Satyanarayana A, Manns MP, Rudolph KL. Telomeres and telomerase: a dual role in hepatocarcinogenesis. Hepatology. 2004;40:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology. 2004;127:S56-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 333] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Ming L, Thorgeirsson SS, Gail MH, Lu P, Harris CC, Wang N, Shao Y, Wu Z, Liu G, Wang X. Dominant role of hepatitis B virus and cofactor role of aflatoxin in hepatocarcinogenesis in Qidong, China. Hepatology. 2002;36:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 539] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 27. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Yano Y, Yamashita F, Sumie S, Ando E, Fukumori K, Kiyama M, Oyama T, Kuroki S, Kato O, Yamamoto H. Clinical features of hepatocellular carcinoma seronegative for both HBsAg and anti-HCV antibody but positive for anti-HBc antibody in Japan. Am J Gastroenterol. 2002;97:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 261] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 31. | Nakamoto Y, Kaneko S, Fan H, Momoi T, Tsutsui H, Nakanishi K, Kobayashi K, Suda T. Prevention of hepatocellular carcinoma development associated with chronic hepatitis by anti-fas ligand antibody therapy. J Exp Med. 2002;196:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 2030] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 33. | Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 496] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 34. | Chen CJ, Chen DS. Interaction of hepatitis B virus, chemical carcinogen, and genetic susceptibility: multistage hepatocarcinogenesis with multifactorial etiology. Hepatology. 2002;36:1046-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Di Bisceglie AM. Screening for hepatocellular carcinoma: being old is not all bad. Am J Gastroenterol. 2004;99:1477-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Talwalkar JA, Gores GJ. Diagnosis and staging of hepatocellular carcinoma. Gastroenterology. 2004;127:S126-S132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 38. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2915] [Article Influence: 108.0] [Reference Citation Analysis (1)] |
| 39. | Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 40. | Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127:S248-S260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1277] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 42. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 539] [Article Influence: 20.7] [Reference Citation Analysis (10)] |
| 43. | Kubo S. Neuer Marker für Leberversagen nach Resektion. Ann Surg. 2004;239:186-193 DOI : 10.1097/01.sla.0000109152.48425.4d PMCid: PMC1356211. |
| 44. | Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261-S267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Schwartz M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S268-S276. [PubMed] |
| 47. | Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S277-S282. [PubMed] |
| 48. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5388] [Article Influence: 179.6] [Reference Citation Analysis (7)] |
| 49. | Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, Rosen M, Soulen M, Shaked A, Reddy KR. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Sala M, Varela M, Bruix J. Selection of candidates with HCC for transplantation in the MELD era. Liver Transpl. 2004;10:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 704] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 52. | Locker J. A new way to look at liver cancer. Hepatology. 2004;40:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 54. | Cronin DC, Millis JM, Siegler M. Transplantation of liver grafts from living donors into adults--too much, too soon. N Engl J Med. 2001;344:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 329] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 56. | Lo CM, Fan ST, Liu CL, Chan SC, Wong J. The role and limitation of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2004;10:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Livraghi T, Meloni F, Morabito A, Vettori C. Multimodal image-guided tailored therapy of early and intermediate hepatocellular carcinoma: long-term survival in the experience of a single radiologic referral center. Liver Transpl. 2004;10:S98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 59. | Head HW, Dodd GD. Thermal ablation for hepatocellular carcinoma. Gastroenterology. 2004;127:S167-S178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 604] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 61. | Lencioni R, Pinto F, Armillotta N, Bassi AM, Moretti M, Di Giulio M, Marchi S, Uliana M, Della Capanna S, Lencioni M. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: a European experience. Eur Radiol. 1997;7:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 63. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 64. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 65. | Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the "test-of-time approach". Cancer. 2003;97:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 66. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2008] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 67. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 68. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2301] [Article Influence: 100.0] [Reference Citation Analysis (1)] |
| 69. | Llovet JM, Bruix J. Unresectable hepatocellular carcinoma: meta-analysis of arterial embolization. Radiology. 2004;230:300-31; author reply 300-301;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC, Ye QH, Qin LX, Wu ZQ, Fan J, Tang ZY. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10:2791-2794. [PubMed] |
| 71. | Risse JH, Grünwald F, Kersjes W, Strunk H, Caselmann WH, Palmedo H, Bender H, Biersack HJ. Intraarterial HCC therapy with I-131-Lipiodol. Cancer Biother Radiopharm. 2000;15:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189-S193. [PubMed] |
| 73. | Fuss M, Salter BJ, Herman TS, Thomas CR. External beam radiation therapy for hepatocellular carcinoma: potential of intensity-modulated and image-guided radiation therapy. Gastroenterology. 2004;127:S206-S217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG, Goin KA, Van Buskirk M, Roberts CA, Goin JE. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology. 2004;127:S194-S205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 75. | Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8:117-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 280] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 76. | Mathurin P, Rixe O, Carbonell N, Bernard B, Cluzel P, Bellin MF, Khayat D, Opolon P, Poynard T. Review article: Overview of medical treatments in unresectable hepatocellular carcinoma--an impossible meta-analysis? Aliment Pharmacol Ther. 1998;12:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Chow PK, Tai BC, Tan CK, Machin D, Win KM, Johnson PJ, Soo KC. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology. 2002;36:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Yuen MF, Poon RT, Lai CL, Fan ST, Lo CM, Wong KW, Wong WM, Wong BC. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology. 2002;36:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 80. | Yang TS, Lin YC, Chen JS, Wang HM, Wang CH. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2000;89:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 81. | Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783-789. [PubMed] |
| 82. | Palmieri G, Biondi E, Morabito A, Rea A, Gravina A, Bianco A. Thymostimulin treatment of hepatocellular carcinoma on liver cirrhosis. Int J Oncol. 1996;8:827-832. [PubMed] |
| 83. | Stefanini GF, Foschi FG, Castelli E, Marsigli L, Biselli M, Mucci F, Bernardi M, Van Thiel DH, Gasbarrini G. Alpha-1-thymosin and transcatheter arterial chemoembolization in hepatocellular carcinoma patients: a preliminary experience. Hepatogastroenterology. 1998;45:209-215. [PubMed] |
| 84. | Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 268] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 85. | Hsu C, Chen CN, Chen LT, Wu CY, Yang PM, Lai MY, Lee PH, Cheng AL. Low-dose thalidomide treatment for advanced hepatocellular carcinoma. Oncology. 2003;65:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Villa E, Ferretti I, Grottola A, Buttafoco P, Buono MG, Giannini F, Manno M, Bertani H, Dugani A, Manenti F. Hormonal therapy with megestrol in inoperable hepatocellular carcinoma characterized by variant oestrogen receptors. Br J Cancer. 2001;84:881-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Mohr L, Geissler M, Blum HE. Gene therapy for malignant liver disease. Expert Opin Biol Ther. 2002;2:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Geissler M, Mohr L, Ali MY, Grimm CF, Ritter M, Blum HE. Immunobiology and gene-based immunotherapy of hepatocellular carcinoma. Z Gastroenterol. 2003;41:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Mohr L, Yeung A, Aloman C, Wittrup D, Wands JR. Antibody-directed therapy for human hepatocellular carcinoma. Gastroenterology. 2004;127:S225-S231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232-S241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Pei Z, Chu L, Zou W, Zhang Z, Qiu S, Qi R, Gu J, Qian C, Liu X. An oncolytic adenoviral vector of Smac increases antitumor activity of TRAIL against HCC in human cells and in mice. Hepatology. 2004;39:1371-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 92. | Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. 2002;62:3909-3913. [PubMed] |
| 93. | Heathcote EJ. Prevention of hepatitis C virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S294-S302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 94. | Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1204] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 96. | Young MD, Schneider DL, Zuckerman AJ, Du W, Dickson B, Maddrey WC. Adult hepatitis B vaccination using a novel triple antigen recombinant vaccine. Hepatology. 2001;34:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Chen D, Weis KF, Chu Q, Erickson C, Endres R, Lively CR, Osorio J, Payne LG. Epidermal powder immunization induces both cytotoxic T-lymphocyte and antibody responses to protein antigens of influenza and hepatitis B viruses. J Virol. 2001;75:11630-11640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Arntzen CJ. Pharmaceutical foodstuffs--oral immunization with transgenic plants. Nat Med. 1998;4:502-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki AB. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999;13:1796-1799. [PubMed] |
| 100. | Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18:1167-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Davis HL, Mancini M, Michel ML, Whalen RG. DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost. Vaccine. 1996;14:910-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 102. | Prince AM, Whalen R, Brotman B. Successful nucleic acid based immunization of newborn chimpanzees against hepatitis B virus. Vaccine. 1997;15:916-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 399] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 104. | Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatitis C virus-like particles induce virus-specific humoral and cellular immune responses in mice. Hepatology. 2001;34:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Lee AY, Manning WC, Arian CL, Polakos NK, Barajas JL, Ulmer JB, Houghton M, Paliard X. Priming of hepatitis C virus-specific cytotoxic T lymphocytes in mice following portal vein injection of a liver-specific plasmid DNA. Hepatology. 2000;31:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 106. | Weiner AJ, Paliard X, Selby MJ, Medina-Selby A, Coit D, Nguyen S, Kansopon J, Arian CL, Ng P, Tucker J. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J Virol. 2001;75:7142-7148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Brinster C, Muguet S, Lone YC, Boucreux D, Renard N, Fournillier A, Lemonnier F, Inchauspé G. Different hepatitis C virus nonstructural protein 3 (Ns3)-DNA-expressing vaccines induce in HLA-A2.1 transgenic mice stable cytotoxic T lymphocytes that target one major epitope. Hepatology. 2001;34:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Forns X, Payette PJ, Ma X, Satterfield W, Eder G, Mushahwar IK, Govindarajan S, Davis HL, Emerson SU, Purcell RH. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Wedemeyer H, Gagneten S, Davis A, Bartenschlager R, Feinstone S, Rehermann B. Oral immunization with HCV-NS3-transformed Salmonella: induction of HCV-specific CTL in a transgenic mouse model. Gastroenterology. 2001;121:1158-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, Pastore G, Dietrich M, Trautwein C, Manns MP. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 499] [Article Influence: 20.0] [Reference Citation Analysis (1)] |
| 111. | Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung MC, Ulsenheimer A, Schraut WW, Schirren CA, Waechtler M, Backmund M. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 112. | Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, Ishibashi H, Kashiwagi S. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004;39:1213-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 113. | Malik AH, Lee WM. Chronic hepatitis B virus infection: treatment strategies for the next millennium. Ann Intern Med. 2000;132:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 114. | Torresi J, Locarnini S. Antiviral chemotherapy for the treatment of hepatitis B virus infections. Gastroenterology. 2000;118:S83-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 115. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 516] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 116. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1748] [Article Influence: 79.5] [Reference Citation Analysis (1)] |
| 117. | Wands JR. Prevention of hepatocellular carcinoma. N Engl J Med. 2004;351:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 862] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 119. | EASL International Consensus Conference on Hepatitis C. Paris, 26-28, February 1999, Consensus Statement. European Association for the Study of the Liver. J Hepatol. 1999;30:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 410] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 120. | Davis GL. Current therapy for chronic hepatitis C. Gastroenterology. 2000;118:S104-S114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 121. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4559] [Article Influence: 182.4] [Reference Citation Analysis (4)] |
| 122. | Di Bisceglie AM, Hoofnagle JH. Optimal therapy of hepatitis C. Hepatology. 2002;36:S121-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Hadziyannis SJ, Sette H, Jr . Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr., Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355 DOI : 10.7326/0003-4819-140-5-200403020-00010. |
| 124. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 782] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 125. | Nishiguchi S, Shiomi S, Nakatani S, Takeda T, Fukuda K, Tamori A, Habu D, Tanaka T. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001;357:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 179] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 126. | Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Hayashi K. Effect of the dose and duration of interferon-alpha therapy on the incidence of hepatocellular carcinoma in noncirrhotic patients with a nonsustained response to interferon for chronic hepatitis C. Oncology. 2001;61:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 127. | Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 479] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 129. | Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Yamazaki O, Shiomi S, Tamori A, Oka H, Igawa S. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann Intern Med. 2001;134:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 130. | Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 131. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomized trial. Lancet. 2000;356:802-807 DOI : 10.1016/S0140-6736(00)02654-4. |
| 132. | Lau WY, Leung TW, Ho SK, Chan M, Machin D, Lau J, Chan AT, Yeo W, Mok TS, Yu SC. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 285] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 133. | Boucher E, Corbinais S, Rolland Y, Bourguet P, Guyader D, Boudjema K, Meunier B, Raoul JL. Adjuvant intra-arterial injection of iodine-131-labeled lipiodol after resection of hepatocellular carcinoma. Hepatology. 2003;38:1237-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK