Published online Dec 7, 2005. doi: 10.3748/wjg.v11.i45.7183
Revised: April 15, 2005
Accepted: April 18, 2005
Published online: December 7, 2005
AIM: To evaluate the effects of Helicobacter pylori infection on gastric epithelial cell kinetics in patients with chronic renal failure (CRF).
METHODS: Forty-four patients were enrolled in this study and divided into four groups with respect to their Helicobacter pylori (H pylori) and CRF status. Groups were labeled as follows: 1a: normal renal function, H pylori negative (n = 12), 1b: normal renal function, H pylori positive (n = 11), 2a: CRF, H pylori negative (n = 10), 2b: CRF, H pylori positive (n = 11). Upper gastrointestinal endoscopy was done in all the patients involved in the study. During endoscopical investigation, antral biopsy specimens were taken from each patient. In order to evaluate the cell apoptosis and proliferation in gastric epithelial cells, Bax and proliferating cell nuclear antigen (PCNA) labeling indexes (LI) were assessed with immunohistochemical staining method.
RESULTS: For groups 1a, 1b, 2a, and 2b, mean Bax LI was identified as 34.4±13.7, 44.1±16.5, 46.3±20.5, 60.7±13.8, respectively and mean PCNA LI was identified as 36.2±17.2, 53.6±25.6, 59.5±25.6, 67.2±22, respectively. When the one-way ANOVA test was applied, statistically significant differences were detected between the groups for both Bax LI (P = 0.004 <0.01) and PCNA LI (P = 0.009 <0.01). When groups were compared further in terms of Bax LI and PCNA LI with Tukey’s HSD test for multiple pairwise comparisons, statistically significant difference was observed only between groups 1a and 2b (P = 0.006 <0.01).
CONCLUSION: In gastric epithelial cells, expression of both the pre-apoptotic protein Bax and the proliferation marker PCNA increase with H pylori infection. This increase is more evident in patients with uremia. These findings suggest that uremia accelerates apoptosis and proliferation in gastric epithelial cells.
- Citation: Aydemir S, Ozdemir BH, Gur G, Dogan I, Yilmaz U, Boyacioglu S. Effects of Helicobacter pylori infection on gastric epithelial cell kinetics in patients with chronic renal failure. World J Gastroenterol 2005; 11(45): 7183-7187
- URL: https://www.wjgnet.com/1007-9327/full/v11/i45/7183.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i45.7183
Helicobacter pylori (H pylori) is the most common chronic infection in human beings. H pylori infection has been related to peptic ulcer disease, gastric lymphoma and stomach cancers[1,2]. It is an important problem for public health as it can cause several diseases.
Gastrointestinal mucosa is characterized with rapid epithelial cell turnover and homeostasis that is mainly provided by apoptosis[3]. Therefore, it can be considered that the impaired apoptosis may have a role in the pathogenesis of many gastrointestinal system diseases.
In many studies, it has been shown that apoptotic cells are increased after colonization of H pylori in the stomach[4-7], and those increased apoptotic cell levels decrease to normal levels after H pylori eradication[5,8,9]. Increase in apoptotic cell rates causes compensatory hyperproliferative response in order to maintain gastric mucosal tissue mass[10-12].
The mechanisms by which H pylori induces apoptosis of gastric epithelial cells have remained unclear. It has been suggested that many bacterial products might be inducing apoptosis[5,13-15]. One of the factors that has been responsible for apoptosis of gastric epithelial cells is ammonia, which is produced via the degradation of urea by the urease enzyme of H pylori[5,16]. Because of high intragastric urea concentrations in patients with chronic renal failure (CRF), levels of ammonia produced by H pylori are significantly high[16]. Therefore, disorders associated with excessive ammonia production are more prevalent[17,18].
Although the relationship of H pylori with apoptosis and proliferation in gastric epithelial cells has been mostly studied in cases with normal renal functions, gastric epithelial cell kinetics in patients with CRF still remains unknown. This study aims to investigate the effect of H pylori on gastric epithelial cell kinetics in patients with CRF.
The study included 44 patients, of whom 23 with normal renal function and 21 with CRF who were on hemodialysis treatment.
Those with gastric and/or duodenal ulcers as confirmed by upper gastrointestinal endoscopy were excluded from the study. None of the patients included had a history of gastric surgery. Patients who had been treated for H pylori or who had used any antibiotics, proton-pump inhibitor, H2 receptor blocker or any compound that includes bismuth during the last one month were also excluded from the study.
The CRF patients were on a thrice-weekly hemodialysis program. The dialysis prescription included 4-5 h of bicarbonate hemodialysis with standard cuprophane membranes (hollow fiber 1-1.2 m2 surface area, Gambro, Sweden).
We divided the patients into four groups based on their H pylori and renal function status:
Group 1a: normal renal function, H pylori negative (12 patients)
Group 1b: normal renal function, H pylori positive (11 patients)
Group 2a: CFR, H pylori negative (10 patients)
Group 2b: CRF, H pylori positive (11 patients)
All patients were examined by using the upper gastrointestinal endoscopy (video-endoscope Olympus GIP Q240) after premedication with intravenous midazolam (2.5-7.5 mg), after an overnight fast.
During upper gastrointestinal endoscopy, multiple biopsy materials were taken from the antral region of the stomach. The biopsy specimens were transferred to the pathology laboratory in 10% buffered formalin. This study was conducted after getting permission from the local ethics committee of the Baskent University School of Medicine, Ankara, Turkey.
H pylori was detected under microscopy on the histological sections stained with Giemsa staining method.
After a 12-h fasting period, venous blood samples were taken from the forearm-superficial veins of the patients in the morning. In the HD patients, the blood samples were taken before HD session. Serum levels of creatinine (normal <1.2 mg/dL) and blood urea nitrogen (BUN, normal <20 mg/dL) were measured in the central laboratory of our hospital by using routine automated techniques.
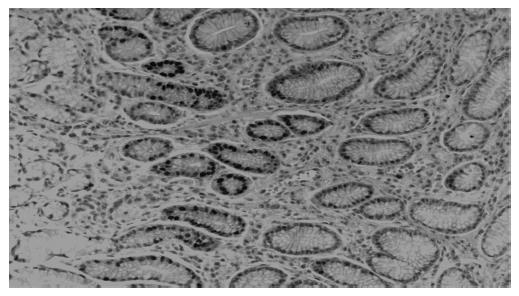
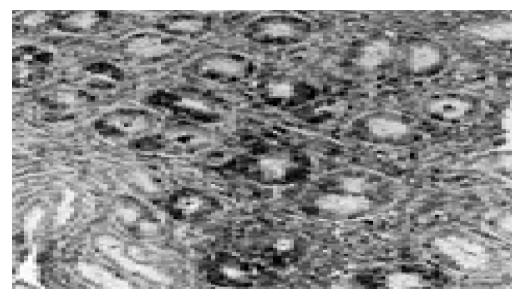
All biopsies were fixed in formalin, embedded in paraffin and processed routinely. Briefly, 4-mm-thick sections were deparaffinized and mounted on poly-L-lysine-coated slides. The sections in a citrate buffer (0.01 mol/L, pH 6) were heated in a microwave oven for 15 min at a maximum power (700 W), and then cooled at room temperature for 20 min. A standard three-step immunoperoxidase avidin-biotin peroxidase complex (ABC) technique was used to identify the proliferating cell nuclear antige (PCNA) (PC10, Neomarkers, CA, USA) (Figure 1). A catalyzed signal amplification (CSA) system was used to detect the polyclonal antibody Bax (K1500, Dako, Denmark) (Figure 2). PCNA and Bax positive cells were counted in the glandular neck region, which corresponds to the area of cell proliferation[19,20]. The field to be counted was chosen under ×400 magnification from the well-labeled area. About 1 000 cells were counted in each case to determine the PCNA and Bax labeling indexes (LI). The percentage of positively stained cells over total cells counted was then calculated and used as a labeling index for PCNA and Bax expression[21,22]. All histological slides were reviewed by the same experienced pathologist. The pathologist who rated the PCNA and Bax LI was unaware of the H pylori and renal function status of the patients.
Statistical analyses were carried out using the program SPSS 9.0 for Windows. To compare the groups, we used Student’s t-test, Mann-Whitney U test, one-way ANOVA and Tukey’s HSD test when appropriate. P values <0.05 were considered statistically significant.
The number of patients, their mean ages and HD duration are shown in Table 1 for each group separately. There were no statistically significant differences between the groups with regard to mean ages, gender, and HD duration (P>0.05).
| Group 1a | Group 1b | Group 2a | Group 2b | |
| Patients (n) | 12 | 11 | 10 | 11 |
| Sex (M/F) | 7/5 | 6/5 | 5/5 | 6/5 |
| Mean age (yr) | 37±7 | 39±7 | 35±8 | 36±11 |
| Hemodialysis | – | – | 10.4±4.5 | 9.3±4.4 |
| duration (mo) |
Creatinine levels were 0.7±02, 0.8±0.2, 7.3±1.1, 7.1±1.3 mg/dL, and BUN levels were 13.8±3.5, 15.4±4.1, 98.7±12.2, 96.1±11.5 mg/dL in groups 1a, 1b, 2a, 2b, respectively (Table 2). Considering the creatinine and BUN levels, there were no statistically significant differences between the groups 1a and 1b (P>0.05), and between the groups 2a and 2b (P>0.05) but levels were higher in groups 2a and 2b than 1a and 1b (P<0.001).
For groups 1a, 1b, 2a, 2b, mean Bax LI was identified as 34.4±13.7, 44.1±16.5, 46.3±20.5, 60.7±13.8, respectively, and mean PCNA LI was identified as 36.2±17.2, 53.6±25.6, 59.5±25.6, 67.2±22, respectively.
When the one-way ANOVA test was applied, statistically significant differences were detected between the groups for both Bax LI (P = 0.004 <0.01) and PCNA LI (P = 0.009 <0.01). When groups were compared further in terms of Bax LI and PCNA LI with Tukey’s HSD test for multiple pairwise comparisons, statistically significant difference was observed only between groups 1a and 2b (P = 0.006 <0.01).
In patients with H pylori infection and CRF, Bax LI and PCNA LI were found to be increased. However, the increase was more prominent in patients with CRF compared to patients with H pylori infection.
This is the first study where cell turnover of gastric epithelial cells is investigated in patients with CRF. We have shown that both H pylori infection and uremia cause increases in Bax and PCNA LI in gastric epithelial cells. Other studies in the literature that show H pylori increases the proliferation and apoptosis in gastric epithelial cells in cases with normal renal function support the results of our study. In addition, we also noted that both apoptosis and proliferation in gastric epithelial cells are more increased in patients with CRF.
Maintaining gastric mucosal integrity is a complex biological process[23]. This subject is provided with the balance between programmed cell death, which is also called apoptosis, and epithelial cell proliferation[23-26]. Apoptosis has been accepted as a physiological form of death and is a genetically programmed process where the cell commits suicide[27,28]. Unlike necrosis, dead cells do not cause inflammatory response in apoptosis[28]. Deranged apoptosis has been implicated in carcinogenesis, autoimmune diseases, and various infectious diseases including H pylori infection[5,23,28,29]. Regulation of apoptosis is a complex process. This process includes the activation of various apoptosis-related proteins such as the Bcl-2 family, p53, Fas and its ligand (FasL), and the interleukin-1b-related concerning enzyme (ICE) family[23,30].
There are several methods for evaluating cell division[31]. PCNA is a co-factor of DNA polymerase and mainly determined in late G1 or S phase. There are several evidences that PCNA assessment is a useful tool to evaluate cell proliferation and previous studies have shown that PCNA index correlates with S phase fraction of tumor cells determined by DNA flow cytometry[32-35]. Furthermore, it has been found that PCNA immunostaining correlates with Ki67, the latter marking cells in G1 and G2 phases in addition to those in S phase and also with tymidine labeling index[36] and with bromodeoxyuridine uptake in cancer cell lines[20,37].
Many genes regulate the balance between apoptosis and cell proliferation. The pro-apoptotic protein, Bax, is a member of Bcl-2 gene family and is one of the genes that regulate apoptosis. Bax gene is a tumor suppressor gene and induces apoptosis via encoding Bax protein. It has been suggested that proliferation and apoptosis in the antral mucosa caused by H pylori is associated with the alterations in genes that regulate the process[8,22,38,39]. Konturek et al[23] showed that apoptosis induced by H pylori in gastric epithelial cells is associated with the upregulation of proapoptotic Bax.
In many studies, it has been shown that the number of apoptotic cells in the stomach increase after H pylori colonization[4-6]. In addition, it has also been shown that the apoptotic cell number in stomach decreases to normal after H pylori eradication[5,8,9]. This increase in apoptotic rate causes compensatory hyperproliferative response in order to maintain gastric mucosa tissue mass[11].
In the literature, there are some data which suggest that apoptosis is associated with both bacterial and host factors[13-15,23]. However, the mechanism of apoptosis in H pylori infection is still not clear.
Some of the factors accused within the apoptosis are lipopolysaccharides, ammonia, and monochloramine (NH2Cl), which is a highly toxic substance generated as a result of the reaction of ammonia with neutrophil-derived free radicals[13-15]. There are still debates on the role of cytotoxic agents such as H pylori-CagA and H pylori-VacA in apoptosis. In recent in vivo and in vitro studies, it has been found that H pylori strains can induce apoptosis without any dependency on Cag-A and Vac-A genotype[23]. In addition to the bacterial factors in infections of H pylori, characteristics of the host can also play important roles. Evidences are increasing about the inflammatory reactions that are induced by H pylori in gastric mucosa, especially cytokines such as nitric oxide and TNFα might assist to induce apoptosis[6].
One of the major factors that are responsible for H pylori derived apoptosis is ammonia. H pylori has a strong urease activity and the hydrolysis of urea causes production of high level of ammonia concentration in mucous gel layer[40]. In vitro studies have shown that H pylori derived ammonia causes lesions in insulated gastric epithelial cells. Ammonia concentrations, determined in H pylori infected subjects, cause gastric mucosal lesions[40-42], delay in mucosal improvement[43], and induce apoptosis in gastric epithelial cells[13,44].
Ammonia level, which is produced by H pylori, is controlled by the existence of urea in gastric juice. Patients with CRF have high intragastric urea concentrations and levels of ammonia produced by H pylori are significantly high[16]. Therefore, disorders associated with excessive ammonia production may be more prevalent in patients with uremia. In our cases, increased apoptosis and proliferation rate that are observed in uremia patients can be associated with excessive ammonia production by H pylori infection in uremia patients compared with cases that have normal renal functions.
In conclusion, in gastric epithelial cells, expression of both the pre-apoptotic protein Bax and the proliferation marker PCNA increase with H pylori infection. This increase is more evident in patients with uremia. These findings suggest that uremia accelerates apoptosis and proliferation in gastric epithelial cells.
| 1. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 483] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Malaty HM, Nyren O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2003;8 Suppl 1:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Brenes F, Ruiz B, Correa P, Hunter F, Rhamakrishnan T, Fontham E, Shi TY. Helicobacter pylori causes hyperproliferation of the gastric epithelium: pre- and post-eradication indices of proliferating cell nuclear antigen. Am J Gastroenterol. 1993;88:1870-1875. [PubMed] |
| 4. | Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695-1703. [PubMed] |
| 5. | Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 315] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238-3243. [PubMed] |
| 7. | Martin JH, Potthoff A, Ledig S, Cornberg M, Jandl O, Manns MP, Kubicka S, Flemming P, Athmann C, Beil W. Effect of H. pylori on the expression of TRAIL, FasL and their receptor subtypes in human gastric epithelial cells and their role in apoptosis. Helicobacter. 2004;9:371-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Nardone G, Staibano S, Rocco A, Mezza E, D'armiento FP, Insabato L, Coppola A, Salvatore G, Lucariello A, Figura N. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Beales IL. Effect of interlukin-1beta on proliferation of gastric epithelial cells in culture. BMC Gastroenterol. 2002;2:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Anti M, Armuzzi A, Gasbarrini A, Gasbarrini G. Importance of changes in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut. 1998;43 Suppl 1:S27-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Suzuki H, Ishii H. Role of apoptosis in Helicobacter pylori-associated gastric mucosal injury. J Gastroenterol Hepatol. 2000;15 Suppl:D46-D54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Hagen SJ, Takahashi S, Jansons R. Role of vacuolation in the death of gastric epithelial cells. Am J Physiol. 1997;272:C48-C58. [PubMed] |
| 14. | Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. bcl-2 deficiency in mice leads to pleiotropic abnormalities: accelerated lymphoid cell death in thymus and spleen, polycystic kidney, hair hypopigmentation, and distorted small intestine. Cancer Res. 1995;55:354-359. [PubMed] |
| 15. | Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany BL. Induction of acute gastritis and epithelial apoptosis by Helicobacter pylori lipopolysaccharide. Scand J Gastroenterol. 1997;32:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | el Nujumi AM, Rowe PA, Dahill S, Dorrian CA, Neithercut WD, McColl KE. Role of ammonia in the pathogenesis of the gastritis, hypergastrinaemia, and hyperpepsinogenaemia I caused by Helicobacter pylori infection. Gut. 1992;33:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Lieber CS, Lefevre A. Ammonia as a source of gastric hypoacidity in patients with uremia. J Clin Invest. 1959;38:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Suzuki H, Yanaka A, Shibahara T, Matsui H, Nakahara A, Tanaka N, Muto H, Momoi T, Uchiyama Y. Ammonia-induced apoptosis is accelerated at higher pH in gastric surface mucous cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G986-G995. [PubMed] |
| 19. | Correa P, Ruiz B, Shi TY, Janney A, Sobhan M, Torrado J, Hunter F. Helicobacter pylori and nucleolar organizer regions in the gastric antral mucosa. Am J Clin Pathol. 1994;101:656-660. [PubMed] |
| 20. | Rokkas T, Liatsos C, Karameris A, Petridou E, Lazaris A, Antoniades D, Kalafatis E. Proliferating cell nuclear antigen (PCNA) immunostaining in Helicobacter pylori infection: impact of eradication. Pathol Oncol Res. 1999;5:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Havard TJ, Sarsfield P, Wotherspoon AC, Steer HW. Increased gastric epithelial cell proliferation in Helicobacter pylori associated follicular gastritis. J Clin Pathol. 1996;49:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Xia HH, Zhang GS, Talley NJ, Wong BC, Yang Y, Henwood C, Wyatt JM, Adams S, Cheung K, Xia B. Topographic association of gastric epithelial expression of Ki-67, Bax, and Bcl-2 with antralization in the gastric incisura, body, and fundus. Am J Gastroenterol. 2002;97:3023-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Konturek PC, Pierzchalski P, Konturek SJ, Meixner H, Faller G, Kirchner T, Hahn EG. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol. 1999;34:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. [PubMed] |
| 25. | Watson AJ. Necrosis and apoptosis in the gastrointestinal tract. Gut. 1995;37:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Han SU, Kim YB, Joo HJ, Hahm KB, Lee WH, Cho YK, Kim DY, Kim MW. Helicobacter pylori infection promotes gastric carcinogenesis in a mice model. J Gastroenterol Hepatol. 2002;17:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9960] [Cited by in RCA: 10157] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 28. | Que FG, Gores GJ. Cell death by apoptosis: basic concepts and disease relevance for the gastroenterologist. Gastroenterology. 1996;110:1238-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4724] [Cited by in RCA: 4704] [Article Influence: 151.7] [Reference Citation Analysis (18)] |
| 30. | Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Unger Z, Molnár B, Szaleczky E, Törgyekes E, Müller F, Zágoni T, Tulassay Z, Prónai L. Effect of Helicobacter pylori infection and eradication on gastric epithelial cell proliferation and apoptosis. J Physiol Paris. 2001;95:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Liu WZ, Zheng X, Shi Y, Dong QJ, Xiao SD. Effect of Helicobacter pylori infection on gastric epithelial proliferation in progression from normal mucosa to gastriccarcinoma. World J Gastroenterol. 1998;4:246-248. [PubMed] |
| 33. | Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779-782. [PubMed] |
| 34. | Garcia RL, Coltrera MD, Gown AM. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989;134:733-739. [PubMed] |
| 35. | Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1105] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 36. | Battersby S, Anderson TJ. Correlation of proliferative activity in breast tissue using PCNA/cyclin. Hum Pathol. 1990;21:781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Coltrera MD, Gown AM. PCNA/cyclin expression and BrdU uptake define different subpopulations in different cell lines. J Histochem Cytochem. 1991;39:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Chen G, Sordillo EM, Ramey WG, Reidy J, Holt PR, Krajewski S, Reed JC, Blaser MJ, Moss SF. Apoptosis in gastric epithelial cells is induced by Helicobacter pylori and accompanied by increased expression of BAK. Biochem Biophys Res Commun. 1997;239:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15-17. [PubMed] |
| 40. | Graham DY, Go MF, Evans DJ. Review article: urease, gastric ammonium/ammonia, and Helicobacter pylori--the past, the present, and recommendations for future research. Aliment Pharmacol Ther. 1992;6:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Murakami M, Saita H, Teramura S, Dekigai H, Asagoe K, Kusaka S, Kita T. Gastric ammonia has a potent ulcerogenic action on the rat stomach. Gastroenterology. 1993;105:1710-1715. [PubMed] |
| 42. | Takeuchi K, Ohuchi T, Harada H, Okabe S. Irritant and protective action of urea-urease ammonia in rat gastric mucosa. Different effects of ammonia and ammonium ion. Dig Dis Sci. 1995;40:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Suzuki H, Yanaka A, Muto H. Luminal ammonia retards restitution of guinea pig injured gastric mucosa in vitro. Am J Physiol Gastrointest Liver Physiol. 2000;279:G107-G117. [PubMed] |
| 44. | Smoot DT, Mobley HL, Chippendale GR, Lewison JF, Resau JH. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990;58:1992-1994. [PubMed] |
Co-first-authors: Selim Aydemir and Binnaz Handan Ozdemir
Co-correspondent: Ibrahim Dogan
Science Editor Wang XL and Guo SY Language Editor Elsevier HK