Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6713
Revised: April 8, 2005
Accepted: April 11, 2005
Published online: November 14, 2005
AIM: To construct the plasmid pcHEV23 containing fragments of HEV ORF2 and ORF3 chimeric gene and to assess its ability to elicit specific immunologic response in mice.
METHODS: The gene encoding the structural protein of HEV ORF2 fragment and full-length ORF3 was amplified by PCR. The PCR products were cloned into an eucaryotic expression plasmid pcDNA3. The resulting plasmid pcHEV23 was used as a DNA vaccine to inoculate BALB/c mice intramuscularly thrice at a dose of 100 or 200 μg. Mice injected with empty pcDNA3 DNA or saline served as control and then specific immune responses in the mice were detected.
RESULTS: After 2-3 times of inoculation, all mice injected with pcHEV23 had anti-HEV IgG seroconversion and specific T lymphocyte proliferation. The lymphocyte stimulation index in the group immunized with pcHEV23 (3.1±0.49) was higher than that in the control group (0.787±0.12, P<0.01). None in the control group had a detectable level of anti-HEV IgG.
CONCLUSION: DNA vaccine containing HEV ORF2 and ORF3 chimeric gene can successfully induce specific humoral and cellular immune response in mice.
- Citation: Hong Y, Ruan B, Yang LH, Chen Y, Jing L, Wang YT, Hu HJ. Hepatitis E virus chimeric DNA vaccine elicits immunologic response in mice. World J Gastroenterol 2005; 11(42): 6713-6715
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6713.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6713
Hepatitis E virus (HEV) is an unclassified, non-enveloped RNA virus, a causative agent of acute hepatitis E transmitted principally via the fecal-oral route. The virus can cause large water-borne epidemics of the disease and sporadic cases as well. Hepatitis E occurs predominantly in developing countries usually affecting young adults with a fatality rate of 15-20% in pregnant women[1]. However, no effective treatment is currently available for hepatitis E and there are no commercial vaccines for hepatitis E in the world. Although at least four major genotypes of HEV have been identified, only one serotype of HEV is recognized. DNA vaccine can synthesize viral proteins within the host cells and induce humoral and cellular immune responses[2,3]. In this study, we constructed eucaryotic expression plasmid containing HEV ORF2 fragment and full-length ORF3 (DNA vaccine) chimeric gene and inoculated it to BALB/c mice to detect the specific humoral and cellular immune responses in mice.
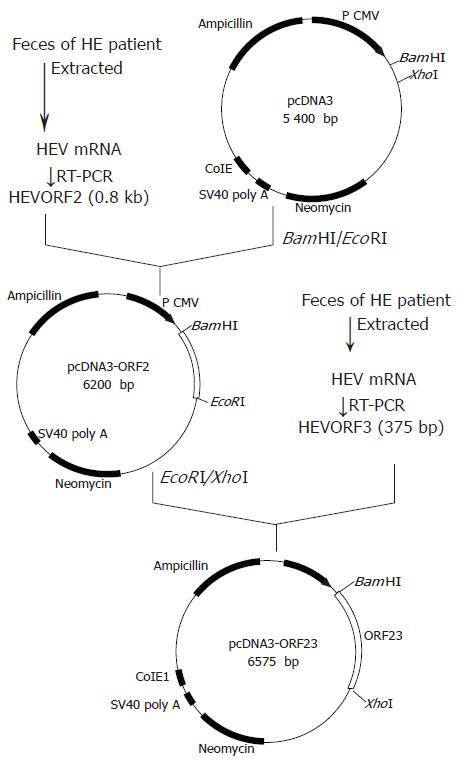
All PCR primers were designed according to the nucleotide sequence of a Chinese HEV isolate (DDBJ accession number D11092)[4]. HEV mRNA was extracted from the feces of a patient with hepatitis E in Hangzhou, Zhejiang Province. The HEV ORF2 fragment and full-length ORF3 chimeric gene were amplified by RT-PCR. The PCR product was inserted into an eucaryotic expression plasmid pcDNA3 to form a recombinant plasmid pcHEV23 (DNA vaccine) (Figure 1).
Thirty-two female BALB/c mice (18-20 g) provided by Zhejiang Experimental Animal Center were used for immunization and divided into four groups: Group 1 was injected with 100 μL saline solution as control. Group 2 was injected with 100 μg/100 μL vector pcDNA3 as control. Group 3 was injected with 100 μg/100 μL pcHEV23 plasmid. Group 4 was injected with 200 μg/100 μL pcHEV23 plasmid. Three weeks after the first injection, mice were bled and then boosted by same method. After another 3 wk, mice were boosted again for the second time (Table 1).
| Group | Mice (n) | Immunogen | Time of injection (wk) |
| 1 | 8 | Saline | 0 3 6 |
| 2 | 8 | pcDNA3(100 μg/100 μL) | 0 3 6 |
| 3 | 8 | pcHEV23(100 μg/100 μL) | 0 3 6 |
| 4 | 8 | pcHEV23(200 μg/100 μL) | 0 3 6 |
Two weeks after the first three injections, blood samples were collected. All serum specimens were tested for anti-HEV IgG by EIA. In brief, microwell plates (Nunc, Roskilde, Denmark) were coated overnight at 4 °C with purified HEV ORF23 proteins (expressed in E coli) at 1 μg/mL in carbonate–bicarbonate buffer (pH 9.6). The wells were washed thrice with 0.05% Tween 20 in PBS (PBS-T) and then blocked with 2% BSA in PBS-T at 37 °C for 1 h. Following three washes with PBS-T, serum samples diluted in 2% BSA were added to the plates and incubated at 37 °C for 1 h. Following five washes, HRP-conjugated anti-human IgG (Sigma) diluted 2 000-fold in PBS-T was added to detect the bound antibodies. Following incubation at 37 °C for 1 h, the plates were washed as above and the substrate tetramethylbenzidine solution (Sigma) was added to the wells. After incubation at room temperature for 15 min, color development was stopped by adding 2 mol/L H2SO4. Optical density (OD) at 450 nm was determined with an ELISA reader. The cut-off values were set for each test as 2.1 times the mean value of negative control samples.
All the animals were killed 14 d after the last injection to analyze cellular immune responses. Single cell suspension of splenocytes was prepared for each individual animal. Splenocytes were immediately cultured in the presence of HEV ORF23 (20 μg/mL, expressed in E coli). Sixty-eighth day after culture, MTT was then added to the cells to measure antigen-specific proliferation. Lymphocyte stimulation index (SI) was measured according to formula: SI = A570 (antigen stimulation)/A570 (control).
The sequencing data indicated that the pcHEV23 construct (Figure 1) contained the correct orientation of the HEV ORF2 and ORF3 fragments (sequencing data not shown).
None of the mice had seroconversion after the initial injection of pcHEV23. Following the third dose of pcHEV23, anti-HEV IgG titers in groups 3 and 4 was higher than those in groups 1 and 2 (P<0.01, Table 2).
| Group | 1 | 2 | 3 | 4 |
| Mean±SD | 0.116±0.009b | 0.210±0.028b | 0.353±0.085b | 0.336±0.066b |
The lymphocyte SI in the group immunized with pcHEV23 was higher than that in saline group and pcDNA3 in control DNA group (P<0.01). Results are shown in Table 3.
| Group | 1 | 2 | 3 | 4 |
| 0.787±0.12b | 1.54±0.25b | 3.1±0.49b | 2.85±0.59b |
DNA vaccines represent a new and potentially powerful approach for the development of subunit vaccines. DNA vaccines induce a broad range of immune responses due to efficient priming of T lymphocytes[5]. This novel approach to vaccination is attractive as it offers several desirable features. First, DNA is not infectious and does not replicate and encodes only the protein or proteins of interest. Second, DNA is stable and can be made inexpensively in large quantities at a high level of purity. Third, plasmid DNA does not contain any heterologous protein components compared to a recombinant virus vaccine. Fourth, DNA vaccine can induce both cell-mediated and humoral immunity. Finally, antigen expression persists after DNA vaccination, promoting the induction of long-lived memory immune cells[6]. DNA immunization can be defined as a physical delivery of nucleic acids in vivo to express antigenic proteins and elicit specific immune responses. Direct naked DNA inoculation obviates the requirement of purified antigens. Pathogenic antigens synthesized inside the inoculated host cells can be processed in a natural form to develop classes I and II-regulated immune responses, mimicking the aspects of live attenuated virus.
Epitope mapping demonstrates that there are at least 7 immunodominant epitopes in HEV ORF2 region and four epitopes in HEV ORF3 region[7,8]. In this study, the full length of HEV ORF3 cDNA and partial HEV ORF2 cDNA were combined and used as an antigen-coding sequence for construction of HEV DNA vaccine.
In conclusion, direct injection of pcHEV23 is able to induce specific anti-HEV IgG and T-lymphocyte proliferation. HEV DNA vaccine constructed by us can successfully induce both humoral and cellular immune responses and appears to be a viable alternative to the recombinant protein subunit vaccine candidate.
| 1. | Wang L, Zhuang H. Hepatitis E: an overview and recent advances in vaccine research. World J Gastroenterol. 2004;10:2157-2162. [PubMed] |
| 2. | Tang DC, DeVit M, Johnston SA. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1022] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 3. | Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2667] [Cited by in RCA: 2803] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 4. | Bi SL, Purdy MA, McCaustland KA, Margolis HS, Bradley DW. The sequence of hepatitis E virus isolated directly from a single source during an outbreak in China. Virus Res. 1993;28:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Sarzotti M, Dean TA, Remington MP, Ly CD, Furth PA, Robbins DS. Induction of cytotoxic T cell responses in newborn mice by DNA immunization. Vaccine. 1997;15:795-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Pardoll DM, Beckerleg AM. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Khudyakov YE, Khudyakova NS, Fields HA, Jue D, Starling C, Favorov MO, Krawczynski K, Polish L, Mast E, Margolis H. Epitope mapping in proteins of hepatitis E virus. Virology. 1993;194:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Khudyakov YuE MO, Jue DL, Hine TK, Fields HA. Immunodominant antigenic regions in a structural protein of the hepatitis E virus. Virology. 1994;198:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK