Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6701
Revised: June 1, 2005
Accepted: June 2, 2005
Published online: November 14, 2005
AIM: To investigate the correlation between tissue ST6Gal I and serum msAFP in HCC patients, and to investigate their prognostic significance.
METHODS: Preoperative sera, paired tumorous and non-tumorous tissues were collected from 19 consecutive patients who had undergone surgical resection of HCC. ST6Gal I activities in the tissues were measured by an in vitro microsomal enzyme activity assay. The percentages of tumor-specific msAFP in the sera were also estimated by an isoelectric focusing-immunoblotting assay.
RESULTS: The tumor ST6Gal I activity was negatively correlated with serum msAFP percentage (r = -0.53, P = 0.019). Both decreased tumor ST6Gal I activity and increased serum msAFP percentage were associated with poor tumor cell differentiation. Univariate analyses showed that both decreased tumor ST6Gal I activity (P = 0.028), increased serum msAFP percentage (P = 0.034) and poor tumor cell differentiation (P = 0.031) were associated with shorter overall survival. Multivariate analysis using the Cox regression model showed that the preoperative serum msAFP percentage (P = 0.022) and tumor cell differentiation status (P = 0.048) were independent prognostic indicators for patient overall survival.
CONCLUSION: Our results indicate that the presence of msAFP in blood circulation is associated with a decreased activity of ST6Gal I activity in HCC. Both tissue ST6Gal I and serum msAFP are potential prognostic markers for patients with operable HCC.
- Citation: Poon TC, Chiu CH, Lai PB, Mok TS, Zee B, Chan AT, Sung JJ, Johnson PJ. Correlation and prognostic significance of beta-galactoside alpha-2,6-sialyltransferase and serum monosialylated alpha-fetoprotein in hepatocellular carcinoma. World J Gastroenterol 2005; 11(42): 6701-6706
- URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6701
Beta-galactoside alpha-2,6-sialyltransferase (ST6Gal I) is the key enzyme for the production of alpha-2,6-linked sialoglycoconjugates. Both ST6Gal I and alpha-2,6-linked sialoglycoconjugates have been suggested to play important roles in oncogenic transformation and metastasis[1-3]. Upregulated expression of ST6Gal I has been shown in colorectal cancer[4,5], breast cancer[6], cervical cancer[7], and choriocarcinoma[8]. However, elevated alpha-2,6 sialylation inhibited formation of glioma in vivo[9]. Expression of ST6Gal I may have different effects in different cancer types.
Serum alpha-fetoprotein (AFP) is a conventional marker for the diagnosis of hepatocellular carcinoma. Eighty to ninety percent of patients with HCC will have levels above the reference range[10-12]. A serum concentration >500 μg/L, in an area with high incidence of HCC, and in the appropriate clinical setting, is usually considered diagnostic of HCC. However, modestly raised levels of AFP (10-500 μg/L) are also common in non-malignant chronic liver disease, so that the specificity of the AFP test for HCC tends to be low[10,13-15]. Recent studies have shown that monosialylated AFP (msAFP), which is a hyposialylated isoform of alpha-fetoprotein (AFP), is specific to hepatocellular carcinoma (HCC)[16,17]. msAFP can be identified, measured quantitatively by isoelectrofocusing electrophoresis approach or by glycosylation immunosorbent assay[18]. msAFP percentage (msAFP%) relative to total AFP can be used as a serum marker to differentiate HCC patients with non-diagnostic total AFP from patients with chronic liver diseases[18].
Sialylation of AFP is mediated by cellular ST6Gal I. We hypothesize that downregulation of ST6Gal I plays an important role in the HCC pathogenesis, and causes the presence of msAFP in blood circulation of HCC patients. In the present study, we examined the enzyme activity and mRNA expression of ST6Gal I in the paired tumorous and non-tumorous tissues from patients with HCC. The correlation among ST6Gal I activity, serum msAFP percentage and their clinical implications were investigated.
Between December 1999 and January 2001, 19 consecutive patients who had undergone surgical resection of HCC, which contained viable tumor cells as shown by histological examination, and with serum AFP levels higher than 20 ng/mL were recruited into this study. Informed consent was obtained for using the resected tissues and sera for research studies. The patients were followed up for 2 years or until death. Preoperative sera, paired tumorous and non-tumorous tissues were collected. Sera were stored at -20 °C until further analysis. The tissues were cut into small pieces, snap frozen with liquid nitrogen, and stored at -70 °C. The AFP levels in the serum samples were quantified with a commercial sandwich-type ELISA (DAKO, Glostrup, Denmark). This study was approved by the Joint CUHK-NTEC Clinical Research Ethics Committee.
The tissues were homogenized on ice in 1 mL of sodium phosphate buffer (PBS, 10 mmol/L NaPO4, 150 mmol/L NaCl, pH 7.4), containing 0.8 mmol/L protease inhibitor (Pefabloc® SC PLUS, Boehringer Mannheim, Roche Diagnostics, Germany), with a Polytron-Aggregate (Kinetica, Switzerland) using a 11-mm cutting probe at a speed setting of 5 for 15 s. After centrifugation at 4 °C for 10 min at 1 000 r/min, the supernatant fraction was saved and centrifuged again at 13 000 g for 30 min at 4 °C. The microsomal pellet obtained after centrifugation was then resuspended in Tris-HCl buffer (15 mmol/L Tris-HCl, pH 6.0) and stored at -70 °C until analyzed. The protein content was measured by the Bradford Coomassie Dye-Binding Protein Assay.
The assay was similar to the method described by Pousset et al[19] and Halliday et al[20] It was based on the specific binding property of Sambucus nigra agglutinin (SNA, Calibiochem, San Diego, USA) that preferentially binds sialic acid residues attached to terminal Galβ1, 4GlcNAc units on N-glycan in alpha-2,6, but not 2,3 or 2,8 linkage.
Briefly, the microsomal preparation was used as a source of alpha-2,6-sialyltransferase, and the N-glycan on asialofetuin (ASF) (Sigma, St. Louis, USA) was used as a sialic acid acceptor. The ST6Gal I activity assay was carried out in a mixture containing 10 μg of tissue microsome preparation, 125 μg ASF, 125 μmol/L CMP-sialic acid and 0.25 μCi CMP-[3H]-sialic acid in reaction buffer (0.1% BSA, 0.1% Triton X-100, 45 mmol/L NaCl, 15 mmol/L Tris-HCl, pH 6.0) with a final volume of 20 μL. The mixture was added to 30 μL of SNA-sepharose (1 mg SNA covalently linked to 0.3 g of CNBr activated Sepharose 4B gel), mixed, and incubated at 37 °C overnight. After washing the gels four times with washing buffer (PBS containing 0.5% Tween 20), the molecules bound to the SNA-Sepharose were released by vortexing the gel in 300 μL of 1 mol/L H2SO4 for 30 s. Two hundred and eighty-five microliters of the supernatant was transferred to a vial, and 5 mL scintillant solution was added. The amount of radiolabeled ASF was then measured with a liquid scintillation counter. Commercial rat ST6Gal I (Sigma) was used as the calibration standard. One unit of the commercial rat ST6Gal I is defined as the amount that will transfer 1.0 μmol of sialic acid from CMP-sialic acid to asialomucin per minute at pH 6.5.
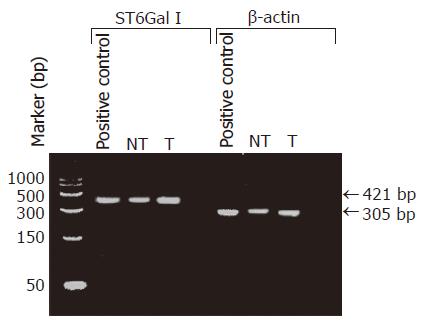
Ten to thirty milligrams of frozen tumorous or non-tumorous liver tissue was disrupted and homogenized using a mortar and pestle (Kontes, Vineland, NJ, USA). Total RNA was extracted from the tissue sample using the RNeasy Mini Kit (Qiagen, GmbH, Germany), and reverse transcribed into cDNA by using a 1st strand cDNA Synthesis Kit (AMV, Roche Diagnostic, Boehringer Mannheim, Germany) with the oligo-p(dT)15 primer provided. The cDNA was then used as the template for PCR amplification with primers specific to ST6Gal I (forward: 5’-CCTGAACAATTCCAGCCT GCTCCTTT-3’ and reverse: 5’-GACGATGTTTCCAATCCCCTGTACCA-3’) or β-actin gene (forward, 5’-CTTCTACAATGAGCTGCGT-3’ and reverse: 5’-TCATGAGGTAGTCAGTCAG-3’). In the PCR for ST6Gal I, the cDNA, diluted in Tris–EDTA buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0), was mixed with 1×PCR buffer, 2 mmol/L DIG-labeled dNTP, 2.5 mmol/L MgCl2, 1 μmol/L of each primer, 0.83 U Taq DNA polymerase, 0.022 μmol/L TaqStart Antibody, 0.8 U uracil glycosylase in a final volume of 20 μL. The mixture was incubated at room temperature for 10 min. Then an initial denaturation step of 2 min at 94 °C was done, followed by repeating cycles of 1 min at 94 °C, 1 min at 63 °C for primer annealing, and 1 min at 72 °C for extension. A final extension run of 7 min at 72 °C was performed to ensure complete elongation of all amplicons. In the PCR for β-actin gene, 3.5 mmol/L of MgCl2 was used instead of 2.5 mmol/L, and the primer annealing temperature in each PCR cycle was 60 °C instead of 63 °C. In the initial attempts, 40 cycles of PCR were performed. The specificity of the PCR amplication was checked by agarose gel electrophoresis to see if the sizes of the amplicons were the same as the expected values, 421 bp for ST6Gal I cDNA and 305 bp for β-actin gene. A commercially available cDNA preparation of human normal liver tissues, pooled from two male/female Caucasians, was used as positive control (Clontech Laboratories, Inc., CA, USA).
For the semi-quantitative assay, 25 cycles of PCR were performed in the presence of DIG-labeled dNTP. The concentrations of the DIG-labeled amplicons were measured with the PCR ELISA (DIG-detection) kit (Roche Diagnostics), according to the manufacturer’s instruction. Briefly, the DIG-labeled amplicons were first denatured, and hybridized with 7.5 pmol biotinylated DNA probe specific for the ST6Gal I (biotin-5’-TGCATTGGGC ACAATTGTAA-3’) or β-actin gene (biotin-5’-GTCCAGACGCAGGATGGCAT-3’). The DNA-probe hybrids were then captured onto an avidin-coated microplate. After washing, the amount of the DNA-probe hybrids bound to the microplate was determined by incubating with anti-DIG-HRP conjugate at 37 °C for 30 min, followed by adding the BM Blue peroxidase substrate (Roche Diagnostics). One hundred microliters of 1 mol/L H2SO4 was added to stop the colorimetric reaction. The optical density of the wells was determined at 450 nm with a reference wavelength of 690 nm. The ST6Gal I/β-actin mRNA ratios were calculated as the mRNA values of the tissue ST6Gal I.
The semi-quantitative analysis of msAFP was performed as previously reported by us[18]. AFP isoforms in the serum samples were separated by IEF on a polyacrylamide gel, which were pre-swollen with a solution containing 5 mol/L urea and 1:16 Pharmalyte 4.5-5.4 (Amersham Pharmacia Biotech, Uppsala, Sweden) in double distilled water. One microliter of pre-diluted serum samples or standard containing 5 or 10 ng/mL AFP. The total AFP was applied to the anode side of the gel after prefocusing (2 000 V, 2.0 mA, 3.5 W, 10 °C, 75 V.h). The sample was applied for 15 V.h (200 V, 2.0 mA, 3.5 W, 10 °C). The final isoelectric separation step was done for 450 V.h (2 000 V, 5.0 mA, 3.5 W, 10 °C). The focused proteins were transferred to nitrocellulose membrane, and then incubated with polyclonal rabbit anti-human AFP (DAKO), followed by horseradish peroxidase conjugated polyclonal swine anti-rabbit immunoglobulin (DAKO). After washing, the enhanced chemiluminescence detection system (ECL, Amersham Pharmacia Biotech) was used to visualize the AFP protein bands. The image of each band was scanned with a densitometer (GS-700, Bio-Rad, CA, USA), and the intensity of each band was expressed as a percentage of the total intensity of all AFP bands.
The Wilcoxon signed rank test was used to compare the differences between the paired tumor and non-tumor groups. The Mann–Whitney rank sum test and Fisher’s exact test were used to compare the differences between other study groups. Correlations between the study parameters were analyzed by the Spearman’s rank order correlation test. The log-rank test and Cox proportional hazards model were applied for survival analyses.
The clinical features of the 19 patients with primary HCC are summarized in Table 1. The tumor ST6Gal I activity was compared to that of the paired non-tumorous tissue. Seven HCC patients fell into a group with decreased ST6Gal I activity in the tumorous tissue, whereas 12 HCC patients fell into a group with increased ST6Gal I activity in the tumorous tissue (Table 2). The tumor ST6Gal I activities in the decreased group were significantly lower than the values in the increased group (P = 0.005).
| Case | Sex | Age | HBs Ag positivity | Cirrhosis | Tumor stage | Tumor size | Tumor | Encapsulation | Capsule invasion | Vascular invasion | Preoperative serum | |
| ALTSG AJCC | (cm) | differentiation | AFP (ng/mL) | |||||||||
| 1 | F | 51 | Yes | Yes | T2 | T2 | 2.4 | Moderate | Yes | Yes | No | 20 |
| 2 | M | 75 | Yes | Yes | T2 | T2 | 3.4 | Moderate | Yes | Yes | No | 25 |
| 3 | M | 52 | Yes | No | T3 | T2 | 10 | Moderate | Yes | No | No | 64 |
| 4 | M | 44 | Yes | Yes | T3 | T3 | 4 | Well | Yes | Yes | Yes | 69 |
| 5 | M | 44 | No | Yes | T2 | T2 | 2.6 | Poor | Yes | Yes | No | 73 |
| 6 | M | 53 | Yes | Yes | T3 | T2 | 8.3 | Poor | Uncertain | Uncertain | No | 100 |
| 7 | M | 65 | No | Yes | T2 | T2 | 4.4 | Moderate | Yes | No | No | 150 |
| 8 | F | 41 | Yes | Yes | T3 | T2 | 7 | Well | Yes | Yes | No | 151 |
| 9 | M | 57 | No | Yes | T3 | T2 | 6 | Poor | Yes | Yes | Yes | 152 |
| 10 | M | 46 | Yes | Yes | T1 | T1 | 1.2 | Moderate | Yes | Yes | No | 365 |
| 11 | M | 60 | Yes | Yes | T2 | T2 | 2.2 | Poor | Yes | Yes | No | 618 |
| 12 | M | 67 | Yes | Yes | T2 | T2 | 2.5 | Moderate | Yes | Yes | No | 1 057 |
| 13 | M | 48 | Yes | No | T2 | T2 | 4 | Moderate | Yes | No | No | 1 427 |
| 14 | M | 43 | Yes | No | T2 | T2 | 2.5 | Moderate | Yes | Yes | No | 1 500 |
| 15 | F | 31 | Yes | Yes | T2 | T2 | 2 | Moderate | Yes | No | No | 2 726 |
| 16 | M | 40 | Yes | No | T2 | T2 | 1.6 | Moderate | No | No | No | 3 338 |
| 17 | M | 35 | Yes | No | T3 | T2 | 8 | Poor | Yes | No | No | 5 505 |
| 18 | M | 42 | Yes | Yes | T2 | T2 | 3.4 | Poor | Yes | Yes | No | 12 185 |
| 19 | M | 72 | Yes | No | T3 | T3 | 6.5 | Poor | Yes | Yes | No | 42 837 |
| Group 1: decreased ST6Gal | Group 2: increased ST6Gal | P1 | |
| (n = 7) | (n = 12) | ||
| Tumor ST6Gal I activity (mU/mg of protein) | 0.57 (0.47, 0.37-0.59) | 1.76 (1.21, 0.94-1.93) | 0.005 |
| Relative tumor ST6Gal I activity (against non-tumor ST6Gal I activity) | 0.46 (0.42, 0.25-0.71)2 | 2.04 (1.51, 1.35-1.88) | <0.001 |
| Tumor ST6Gal I mRNA level3 | 1.78 (0.64, 0.24-1.7) | 1.51 (1.08, 0.68-2.6) | N.S4 |
| Serum AFP level (ng/mL) | 7687 (680, 246-145045) | 739 (161, 72-1 197) | N.S. |
| Serum msAFP percentage (%) | 35 (41, 23-45) | 18 (13, 11-17) | 0.031 |
The specificity of the RT-PCR reaction was checked by subjecting the RT-PCR product to agarose gel electrophoresis. In all cases including the positive control (normal human liver cDNA), the RT-PCR product appeared as a single DNA band with the expected size of 421 bp for ST6Gal I, or 305 bp for β-actin. The electrophoresis results indicated that the RT-PCR amplifications were specific. Our data also confirmed that ST6Gal I gene was expressed in all the HCC tissues and the non-tumorous liver tissues (Figure 1). Two cases were omitted for the RT-PCR ELISA to measure ST6Gal I mRNA level owing to insufficient tissue materials. The measured ST6Gal I mRNA level of individual tissues was normalized by expressing the data as a ratio of β-actin mRNA level. In the non-tumorous tissues, the ST6Gal I mRNA level positively correlated with the ST6Gal I activity (r = 0.49, P = 0.039). In the tumorous tissues, no significant correlation was found between the enzyme activity (r = -0.093, P = 0.72).
The percentages of tumor-specific msAFP (relative to total intensity of AFP isoforms) in the preoperative sera of the HCC patients were estimated, and the results of the patient groups with increased and decreased tumor ST6Gal I enzyme activity are shown in Table 2. Comparison of the two groups shows that the percentage of msAFP in the patient group with decreased tumor enzyme activity was significantly higher than those in the group with increased activity (P = 0.031), whereas the serum total AFP levels were not significantly different. Furthermore, the msAFP percentage was negatively correlated with the relative tumor ST6Gal I enzyme activity (r = -0.53, P = 0.019).
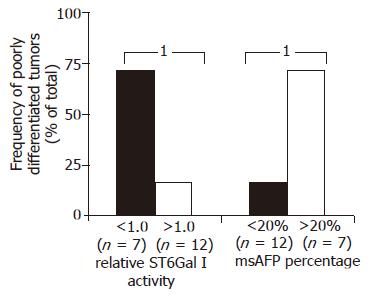
The relationships between ST6Gal I activity, msAFP percentage and various patient clinical parameters, including HBV status, tumor stage (ALTSG and AJCC), tumor size, differentiation status, vascular invasion, encapsulation, capsule invasion, metastasis and recurrent, were investigated. More cases with poorly differentiated tumor were found in the patient groups with decreased enzyme activity (P = 0.045, Figure 2). Similar to tumor ST6Gal I activity, but in an opposite manner, more cases with poorly differentiated tumor were found in the patient group with higher (>20%) msAFP percentage (P = 0.045, Figure 2).
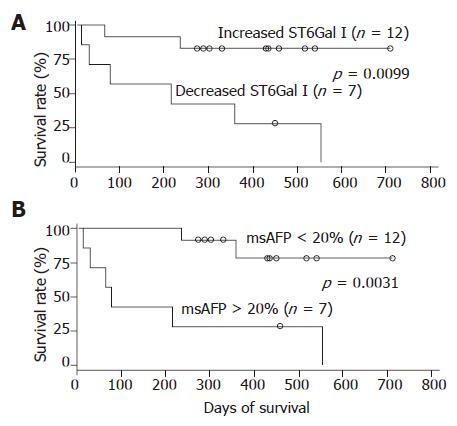
During the 2 years follow-up period, 8 out of the 19 patients died. The absolute/relative tumor ST6Gal I activity, serum msAFP percentage, serum AFP level and various patient clinical parameters were subjected to survival analyses. In univariate analyses using the Cox regression model, the preoperative serum msAFP percentage (P = 0.034), the relative tumor ST6Gal I activity (P = 0.028) and the tumor differentiation status (P = 0.031) were shown to be predictors for patient overall survival. The log-rank test results showed that the patient group with decreased tumor ST6Gal I activity had an overall survival (P = 0.0099, Figure 3A) shorter than the group with increased activity. When the cases were divided into two groups based on the preoperative serum msAFP percentage at a cut-off value of 20%, the log-rank test results showed that higher preoperative serum msAFP percentage was associated with poorer patient overall survival (P = 0.0031, Figure 3B). The log-rank test results also showed that patients with poorly differentiated tumor had poorer overall survival (P = 0.0063). In a multivariate analysis using the Cox regression model, among all the clinical features, only the preoperative serum msAFP percentage (P = 0.022) and tumor cell differentiation status (P = 0.048) were found to be independent prognostic indicators for patient overall survival.
Both ST6Gal I and alpha-2,6-linked sialoglycoconjugates play important roles in oncogenic transformation and metastasis in various cancers[21]. However, information about the influence of ST6Gal I in human HCC tissue has been very limited. The results of the present study were consistent to the findings reported by Cao et al[22] and Dall’Olio et al[23]. Dalli’Olio et al. observed that both ST6Gal I activity can undergo up- or down-regulation in different HCC patients. The present study confirmed Dalli’Olio et al.’s observation. Compared to non-tumorous tissue, the HCC patients could be divided into two groups with increased and decreased ST6Gal I activity in the tumorous tissue. By immunohistochemical staining, Cao et al. showed that expression levels of ST6Gal were decreased in poorly differentiated HCC. This is also consistent to our observation that poorly differentiated HCCs had lower ST6Gal I activities.
The present study provided additional information about ST6Gal I in HCC. We showed that changes of ST6Gal I activity in HCC positively correlated with the presence of msAFP in blood circulation. As sialylation of AFP is mediated by ST6Gal I, probably the presence of hyposialyled AFP variants in blood circulation is caused by decreased ST6Gal I activity. Furthermore, our data suggests that tissue ST6Gal I and serum msAFP are potential prognostic markers for patients with operable HCC.
In the non-tumorous tissues, ST6Gal I mRNA level positively correlated with ST6Gal I activity. This result is consistent to the observation of Svensson et al[24] when studying normal liver. This result therefore could be served as a positive control to confirm the validity of our measurements of tissue ST6Gal I activity and mRNA level. No significant correlation was found in the tumorous tissues. This suggests that ST6Gal I activity in HCC is regulated at post-transcriptional level.
The present study and the studies from Cao et al and Dall’Olio et al[3] strongly indicate that the functional roles of ST6Gal I in HCC are different from those in colorectal cancer and in breast cancer. An in vitro antisense DNA experiment has shown that upregulation of ST6Gal I plays an important role in the invasive potential of human colon carcinoma HT29 cells. Furthermore, high expression levels of ST6Gal I have been correlated with poor survival in colorectal cancer patients[25]. In breast cancers, high ST6Gal I expression was associated with histoprognostic grade III, and negatively correlated to progesterone receptor expression[26]. However, no ST6Gal I expression was found in malignant gliomas or in medulloblastomas[26]. It is worth noting that elevated alpha-2,6 sialylation inhibited formation of glioma in vivo[9]. All these findings indicate that expression of ST6Gal may have different effects in different cancer types.
In conclusion, HCC can be divided into two subtypes, one with decreased tumor ST6Gal I activity and one with increased tumor ST6Gal I activity. The ST6Gal I activity in HCC is not regulated at the transcription level. Downregulation of tumor ST6Gal I activity and an increase in msAFP percentage in preoperative serum are associated with poorly differentiated tumors and poor patient survival. Both ST6Gal I and msAFP percentage are potential prognostic markers for HCC. The presence of msAFP in the blood circulation probably reflects the downregulation of ST6Gal I activity in the HCC tissue.
| 1. | Collard JG, Schijven JF, Bikker A, La Riviere G, Bolscher JG, Roos E. Cell surface sialic acid and the invasive and metastatic potential of T-cell hybridomas. Cancer Res. 1986;46:3521-3527. [PubMed] |
| 2. | Le Marer N, Laudet V, Svensson EC, Cazlaris H, Van Hille B, Lagrou C, Stehelin D, Montreuil J, Verbert A, Delannoy P. The c-Ha-ras oncogene induces increased expression of beta-galactoside alpha-2, 6-sialyltransferase in rat fibroblast (FR3T3) cells. Glycobiology. 1992;2:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Zhu Y, Srivatana U, Ullah A, Gagneja H, Berenson CS, Lance P. Suppression of a sialyltransferase by antisense DNA reduces invasiveness of human colon cancer cells in vitro. Biochim Biophys Acta. 2001;1536:148-160. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Dall'Olio F, Malagolini N, Serafini-Cessi F. Enhanced CMP-NeuAc: Gal beta 1,4GlcNAc-R alpha 2,6 sialyltransferase activity of human colon cancer xenografts in athymic nude mice and of xenograft-derived cell lines. Int J Cancer. 1992;50:325-330. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JV. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354-1367. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Recchi MA, Hebbar M, Hornez L, Harduin-Lepers A, Peyrat JP, Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58:4066-4070. [PubMed] |
| 7. | Wang PH, Li YF, Juang CM, Lee YR, Chao HT, Tsai YC, Yuan CC. Altered mRNA expression of sialyltransferase in squamous cell carcinomas of the cervix. Gynecol Oncol. 2001;83:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Fukushima K, Hara-Kuge S, Seko A, Ikehara Y, Yamashita K. Elevation of alpha2--& gt; 6 sialyltransferase and alpha1--& gt; 2 fucosyltransferase activities in human choriocarcinoma. Cancer Res. 1998;58:4301-4306. [PubMed] |
| 9. | Yamamoto H, Oviedo A, Sweeley C, Saito T, Moskal JR. Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Res. 2001;61:6822-6829. [PubMed] |
| 10. | Johnson PJ, Portmann B, Williams R. Alpha-fetoprotein concentrations measured by radioimmunoassay in diagnosing and excluding hepatocellular carcinoma. Br Med J. 1978;2:661-663. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, Hsu HC, Chuang CN, Yang PC, Wang TH. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259-266. [PubMed] |
| 12. | Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Okuda K. Early recognition of hepatocellular carcinoma. Hepatology. 1986;6:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 211] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570-575. [RCA] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 524] [Article Influence: 21.0] [Reference Citation Analysis (4)] |
| 16. | Johnson PJ, Poon TC, Hjelm NM, Ho CS, Ho SK, Welby C, Stevenson D, Patel T, Parekh R, Townsend RR. Glycan composition of serum alpha-fetoprotein in patients with hepatocellular carcinoma and non-seminomatous germ cell tumour. Br J Cancer. 1999;81:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. Structures of disease-specific serum alpha-fetoprotein isoforms. Br J Cancer. 2000;83:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Poon TC, Mok TS, Chan AT, Chan CM, Leong V, Tsui SH, Leung TW, Wong HT, Ho SK, Johnson PJ. Quantification and utility of monosialylated alpha-fetoprotein in the diagnosis of hepatocellular carcinoma with nondiagnostic serum total alpha-fetoprotein. Clin Chem. 2002;48:1021-1027. [PubMed] |
| 19. | Pousset D, Piller V, Bureaud N, Monsigny M, Piller F. Increased alpha2,6 sialylation of N-glycans in a transgenic mouse model of hepatocellular carcinoma. Cancer Res. 1997;57:4249-4256. [PubMed] |
| 20. | Halliday JA, Franks AH, Ramsdale TE, Martin R, Palant E. A rapid, semi-automated method for detection of Galbeta1-4GlcNAc alpha2,6-sialyltransferase (EC 2.4.99.1) activity using the lectin Sambucus nigra agglutinin. Glycobiology. 2001;11:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Dall'Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconj J. 2001;18:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 212] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Cao Y, Merling A, Crocker PR, Keller R, Schwartz-Albiez R. Differential expression of beta-galactoside alpha2,6 sialyltransferase and sialoglycans in normal and cirrhotic liver and hepatocellular carcinoma. Lab Invest. 2002;82:1515-1524. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Dall'Olio F, Chiricolo M, D'Errico A, Gruppioni E, Altimari A, Fiorentino M, Grigioni WF. Expression of beta-galactoside alpha2,6 sialyltransferase and of alpha2,6-sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology. 2004;14:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Svensson EC, Conley PB, Paulson JC. Regulated expression of alpha 2,6-sialyltransferase by the liver-enriched transcription factors HNF-1, DBP, and LAP. J Biol Chem. 1992;267:3466-3472. [PubMed] |
| 25. | Lise M, Belluco C, Perera SP, Patel R, Thomas P, Ganguly A. Clinical correlations of alpha2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma. 2000;19:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Kaneko Y, Yamamoto H, Kersey DS, Colley KJ, Leestma JE, Moskal JR. The expression of Gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2,6-linked sialoglycoconjugates in human brain tumors. Acta Neuropathol. 1996;91:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Co-first authors: Terence CW Poon and Clarissa HS Chiu
Science Editor Guo SY Language Editor Elsevier HK