Published online Nov 14, 2005. doi: 10.3748/wjg.v11.i42.6644
Revised: May 9, 2005
Accepted: May 12, 2005
Published online: November 14, 2005
AIM: To assess the frequency of herpes simplex virus type I in upper gastrointestinal tract ulcers and normal mucosa with the modern and better assays and also with a larger number of well characterized patients and controls and its relationship to Helicobacter pylori(H pylori).
METHODS: Biopsy specimens from 90 patients (34 with gastric ulcer of the prepyloric area and 56 with duodenal ulcer) were evaluated. Biopsies from 50 patients with endoscopically healthy mucosa were considered as the control group. The method used to identify herpes simplex virus-1 (HSV-1) was polymerase chain reaction. H pylori was detected by the CLO-test and by histological method.
RESULTS: Herpes simplex virus-1 was detected in 28 of 90 patients with peptic ulcer (31%) [11 of 34 patients with gastric ulcer (32.4%) and 17 of 56 with duodenal ulcer (30.4%)] exclusively close to the ulcerous lesion. All control group samples were negative for HSV-1. The likelihood of H pylori negativity among peptic ulcer patients was significantly higher in HSV-1 positive cases than in HSV-1 negative cases (P = 0.009). Gastric ulcer patients with HSV-1 positivity were strongly associated with an increased possibility of Helicobacter pylori negativity compared to duodenal ulcer patients (P = 0.010).
CONCLUSION: HSV-1 is frequent in upper gastro-intestinal tract ulcers but not in normal gastric and duodenal mucosa. There is an inverse association between HSV-1 and H pylori infection.
-
Citation: Tsamakidis K, Panotopoulou E, Dimitroulopoulos D, Xinopoulos D, Christodoulou M, Papadokostopoulou A, Karagiannis I, Kouroumalis E, Paraskevas E. Herpes simplex virus type 1 in peptic ulcer disease: An inverse association with
Helicobacter pylori . World J Gastroenterol 2005; 11(42): 6644-6649 - URL: https://www.wjgnet.com/1007-9327/full/v11/i42/6644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i42.6644
During the last two decades, a significant progress has been made in the role of Helicobacter pylori(H pylori) in the pathogenesis of peptic ulcers, while the invention of new powerful antisecretory drugs has changed dramatically the treatment of the disease. However, the exact etiopathogenesis of peptic ulcer disease is still under investigation.
The significant role of gastric acidity and inflammation of mucosa due to H pylori cannot be disputed, but a multifactorial etiology for peptic ulcer disease seems to be emerging[1-3].
The idea of a possible correlation between HSV-1 and peptic ulcers has appeared almost 40 years before[4,5], due to many common characteristics observed in the clinical picture and the natural history of both diseases[4-7].
A possible involvement of HSV-1 in peptic ulcer disease was reported from several investigators, but a firm conclusion has not yet been reached. The vast majority of these studies are based on the detection of antibodies against the virus in the serum and the duodenal juice of patients with peptic ulcer[8-12], a finding also common in the apparent healthy population. There are only two studies that report the presence of HSV-1 in tissue samples obtained from gastric and duodenal ulcers, using polymerase chain reaction (PCR) methods, but the number of the examined populations is small[13,14]. On the other hand, in the studies reported above, a possible correlation between HSV-1 and H pylori has been investigated in the pathogenesis of peptic ulcer disease.
DNA of HSV-1 has been detected also in human vagal[15] and celiac ganglia[16], which provide the nerve network to gastric tissue. Theoretically, since vagotomy is used to treat peptic ulcer disease, the same treatment may interrupt the migration of activated HSV-1 from ganglia to gastric mucosa, thus preventing the recurrence of ulcer.
The purpose of this study was to investigate the po-ssible relationship between HSV-1 and peptic ulcer and whether viral infection of ulcer patients is related to the presence of H pylori infection.
All patients who underwent esophagogastroduodenoscopy at our institution from September 1999 to September 2002 were recruited. The first group included 56 patients (31 men and 25 women) with active duodenal ulcer (average 53.5±15 years, range from 19 to 83 years). The second group included 34 patients (22 men and 12 women) with active ulcer of the prepyloric area of the stomach (mean of 61.5±16.2 years, range from 22 to 89 years) and the third group that formed the control group, consisted of 50 patients (28 men and 22 women) with no evidence of pathologic findings (mean of 54.8±16.7 years, range from 21 to 86 years).
Tissue samples were taken from all 90 patients with peptic ulcer for the detection of HSV-1 in duplicate, from the following areas: the base and the rim of the ulcer; the adjacent area of the ulcer at a distance of 3 cm (minimal and maximal distance from the crater 3 and 5 cm respectively). For this reason, in the duodenal ulcer group a second duodenal biopsy was obtained; an endoscopically normal area of the stomach (the corpus in gastric ulcer cases and the antrum in duodenal ulcer cases).
Two samples were also taken from endoscopically healthy areas of the antrum and corpus of the stomach in all 50 controls.
Two samples from the antrum and two from the corpus of the stomach were taken for the detection of H pylori using the rapid urease test (CLO-test) and routine histology.
Finally, specimens from the gastric ulcers were exa-mined histopathologically to exclude malignancy.
Risk factors probably involved in the pathogenesis of peptic ulcer, such as non-steroidal anti-inflammatory drugs (NSAIDs), smoking, alcohol, history of herpes labialis, family history of peptic ulcer, and ulcer site, were recorded and analyzed.
Genomic DNA was extracted using the QIAamp DNA mini kit, following the protocol supplied for purification from fresh tissues. DNA was finally dissolved in 50–100 μL of TE buffer depending on the size of DNA pellets and stored at -20 °C until amplification.
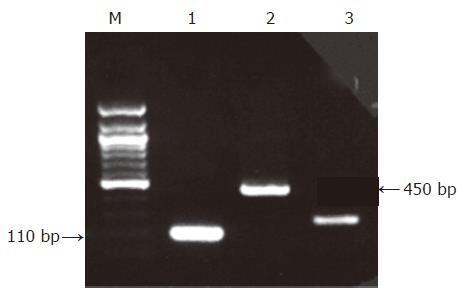
For the nested PCR assay, oligonucleotides deduced from the published sequence of the RL2 gene-coding region from HSV-1 were used[17]. For the control DNA assay, oligonucleotides for b-actin gene were used. The primer sequences and characteristics are shown in Table 1.
| Gene target | GenBankaccession number | Productsize (bp) | Sequences1 | Tm(°C) |
| Outer sense agcagcgactctgaggcggagaccg | 69.1 | |||
| 450 | ||||
| Outer antisense tgcgggtctgggggtcgttcacga | 71.4 | |||
| HSV-1: RL2 | X14112 | Inner sense cccggcagttgcgggggcgc | 73.4 | |
| 110 | ||||
| Inner antisense aaggtgtcgcagcggcaggtg | 60.8 | |||
| b-actin | Forward gtgatctccttctgcatcc | 53.2 | ||
| 200 | 52.7 | |||
| Reverse ctcttccagccttccttc |
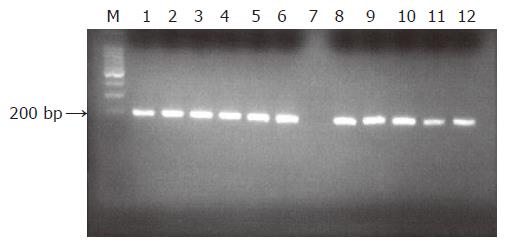
B-actin PCR generating a 200-bp product was performed to determine the DNA integrity of the samples. For the quality control PCR assay, the following program was used 1 cycle at 94 °C for 2 min; 35 cycles at 94 °C for 30 s, at 58 °C for 30 s, at 72 °C for 30 s; and a final cycle at 72 °C for 7 min.
PCR detection of HSV-1 was carried out in a 50 μL reaction mixture containing 25 μL of Taq PCR master mix solution (Qiagen), 13 μL of double-distilled DNase-free water, 1 μmol/L concentration of each primer and 10 μL of the extracted sample. PCR was performed on a PE 9 600 thermocycler (Perkin-Elmer Cetus, Branchburd, NJ, USA). The cycling conditions were at 94 °C for 1 min, 5 cycles at 94 °C for 5 s and at 72 °C for 4 min; 5 cycles at 94 °C for 5 s and at 70 °C for 4 min; 30 cycles at 94 °C for 5 s and at 68 °C for 4 min. After the final cycle, tubes were incubated for an additional 10 min at 72 °C. Nested PCR amplification was done with a 0.5 μL aliquot from the first run, 25 μL of Taq PCR master mix solution, 18 μL of double-distilled DNase-free water and 1 μmol/L concentration of each inner primer under the following cycling conditions: at 94 °C for 2 min; 35 cycles at 90 °C for 30 s, at 68 °C for 30 s, at 72 °C for 30 s and a final extension at 72 °C for 10 min. Each amplification run contained one negative and one positive control. The negative control consisted of blank reagent and water. For the positive control, HSV-1 genomic DNA provided by Sigma was used. Consistent PCR analyses were repeated twice or more.
The PCR products were analyzed by 2% agarose gel electrophoresis in 0.5×Tris–borate EDTA buffer along with ethidium bromide. A molecular weight marker (Φ×174/Hae III, Sigma) was also run simultaneously to identify the molecular size of the PCR products. The DNA bands were visualized by UV transillumination and analyzed using a gel-documentation system. None of the PU and the control samples were negative in the b-actin test ultimately leaving 90 PU and 50 controls that were subjected to HSV-1 PCR analysis.
For the detection of H pylori, a CLO-test was used with high sensitivity and specificity (Kimberly-Clark CLO test, Ballard Medical products, Draper, UT 84020, USA)[18,19].
An experienced pathologist also assessed the his-tological sections with Giemsa stain[20].
All associations between parameters of interest were examined either by Fisher’s exact test or Pearson’s chi square test with continuity correction.
Multivariate analysis was performed using the stepwise logistic regression model to assess the contribution of the common risk factors to peptic ulcer development and H pylori detection.
P<0.05 was considered statistically significant.
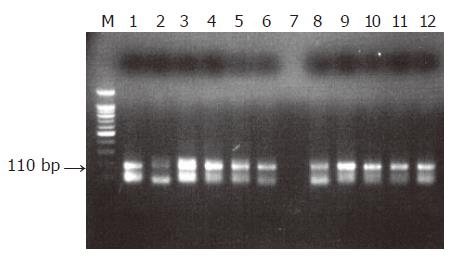
The genome of HSV-1 was present in 28 of the 90 patients with peptic ulcer (31.1%), contrary to the control group in which no positive detections were found (0%, P<0.0005, Figures 1-3). There was an equal prevalence in the two subgroups of patients, 17 of 56 patients with duodenal ulcer (30.4%) and 11 of 34 with gastric ulcer (32.4%) were tested positive (P = 0.843). In all HSV-1 positive cases, the viral genome was detected from the tissue samples obtained from the crater of the ulcer as from the samples obtained from the rim, while all samples from adjacent and distant areas were negative.
All the H pylori positive subjects by CLO test from both groups were also positive for the bacteria with histology.
The incidence of H pylori was significantly higher in peptic ulcer patients (76/90, 84.4%) than in controls (30/50, 60%) (P = 0.002). A statistically significant difference was also found between patients with duodenal ulcer and those with gastric ulcer (P = 0.036). Negative H pylori was more frequently observed in patients with gastric ulcer (26.5%) than in patients with duodenal ulcer (8.9%, Table 2).
| H pylori (+), % | H pylori (–) | P | Odds ratio (95%CI) | |
| Peptic ulcer | 76/90 (84.4) | 14/90 | 0.002 | 3.7 (1.6–8.1) |
| Controls | 30/50 (60) | 20/50 | ||
| Gastric ulcer | 25/34 (73.5) | 9/34 | 0.036 | 3.67 (1.1–12.11) |
| Duodenal ulcer | 51/56 (91.1) | 5/56 | ||
| HSV-1 (+) | 19/28 (67.9) | 9/28 | 0.009 | 5.4 (1.61–18.11) |
| HSV-1 (–) | 57/62 (91.9) | 5/62 | ||
| HSV-1 (+)/GU | 4/11 (36.4) | 7/11 | 0.002 | 18.37 (2.75–122.94) |
| HSV-1 (–)/ GU | 21/23 (91.3) | 2/23 | ||
| HSV-1 (+)/Duodenal ulcer | 15/17 (88.2) | 2/17 | ||
| HSV-1(–)/Duodenal ulcer | 36/39 (92.3) | 3/39 | 0.634 | - |
PCR and CLO test were significantly associated with respect to H pylori detection in all 90 peptic ulcer patients. Negative H pylori was more frequently detected in positive PCR samples (32.1%) than in negative PCR samples (8.1%) (P = 0.009) (Table 2). In the group of duodenal ulcer patients, H pylori negativity was more frequently observed in positive PCR samples (11.8%), than in negative PCR samples (7.7%). However, this difference was not statistically significant (P = 0.634) (Table 2) whereas it was statistically significant in the subgroup of gastric ulcer patients (P = 0.002). In the gastric ulcer subgroup, H pylori, negative cases were observed in 63.6% of positive PCR samples and in 8.7% of negative PCR samples (Table 2). Finally, the likelihood of negative H pylori in HSV-1 positive samples in the group of gastric ulcer patients was significantly higher than that in the group of duodenal ulcer patients (P = 0.010) (Table 3).
| H pylori (+), % | H pylori (-) | P-value | Odds ratio (95% CI) | |
| HSV-1(+)/ gastric ulcer | 4/11 (36.4) | 5/11 | ||
| 0.010 | 13.13 (1.92–89.5) | |||
| HSV-1(–)/ duodenal ulcer | 15/17 (88.2) | 2/17 |
We also studied some of the common risk factors for the development of peptic ulcer disease. No statistically significant difference was found between patients and controls regarding family history of upper gastrointestinal ulcer, history of Herpes labialis, alcohol consumption, and use of NSAIDs (Table 4).
| Peptic ulcer patients (%) | Controls (%) | Odds ratio | P | |
| History of H labialis | ||||
| Yes | 24/90 (26.7) | 9/50 (18.0) | - | 0.302 |
| No | 66/90 (73.3) | 41/50 (82.0) | ||
| Alcohol consumption | ||||
| Yes | 32/90 (35.6) | 14/50 (28.0) | - | 0.469 |
| No | 58/90 (64.4) | 36/50 (72.0) | ||
| NSAID use | ||||
| Yes | 23/90 (25.6) | 8/50 (16.0) | - | 0.275 |
| No | 67/90 (74.4) | 42/50 (84.0) | ||
| Smoking | ||||
| Yes | 45/90 (50.0) | 14/50 (28.0) | 2–57 | 0.019 |
| No | 45/90 (50.0) | 36/50 (72.0) | ||
| Family history of peptic ulcer | ||||
| Yes | 30/90 (33.3) 60/90 (66.7) | 9/50 (18.0) 41/50 (82.0) | 2.3 | 0.076 |
| No |
As expected, tobacco smoking was the only statistically significant risk factor for the development of peptic ulcers between patients and control population (P = 0.019). Smokers were associated with a 2.57-fold increase risk of peptic ulcer development.
The performed multivariate analysis also confirmed that H pylori and tobacco smoking (OR: 3.320 and 2.619 respectively) were more likely to induce peptic ulcer (Table 5).
| B | S.E | df | Sig. | Odds ratio | 95%CI for Odds ratio | |
| Smoking | 0.963 | 0.394 | 1 | 0.015 | 2.619 | 1.209–5.673 |
| H pylori infection | 1.2 | 0.473 | 1 | 0.011 | 3.32 | 1.313–8.389 |
| Age | 0.011 | 0.014 | 1 | 0.432 | ||
| Sex | 0.144 | 0.442 | 1 | 0.745 | ||
| History of H labialis | 0.721 | 0.484 | 1 | 0.137 | ||
| Alcohol | 0.021 | 0.492 | 1 | 0.966 | ||
| NSAID use | 0.286 | 0.494 | 1 | 0.562 | ||
| Family history | 0.44 | 0.48 | 1 | 0.359 | ||
| Constant | –0.741 | 0.393 | 1 | 0.06 |
Despite the progress during the last 20 years in the understanding of the pathogenesis of peptic ulcer disease, it is clear that gastroduodenal ulcer is the result of a multifactorial process.
H pylori infection and NSAIDs have been recognized as the two most important causes of peptic ulcer disease. The proportion of peptic ulcers not associated with H pylori infection or the use of NSAIDs is increasing. Yet several studies have shown that 4.1-44% of peptic ulcers are not related to either of the two factors[21-25].
The possible involvement of HSV-1 in the process is a field of interest for several investigators, but a firm conclusion has not been reached. The presence of viral DNA in tissue samples obtained from the ulcer site is 9.5-18%[13,14]. The possible explanation for this finding is that the HSV-1 expression is prompted either by the ulcer injury or by immune cells[14,16] or HSV-1 itself might cause the ulcerative lesion by directly infecting the mucosal cells[13] or finally that HSV-1 expression is induced by the ulcer treatment[26-28].
In the present study, in a substantially larger number of patients than in the studies reported above (90 vs 22 and 21 respectively), the positivity for HSV-1 DNA was expressed using PCR in a greater percentage of samples (31% vs 9.5% and 18% respectively).
It should be noted that viral DNA was detected only in the tissue samples obtained from the base and the rim of the lesions, whereas all the other examined samples obtained from the adjacent ulcer areas and endoscopically healthy mucosa from the patients and the control group were negative for the viral genome.
This finding can be explained as follows. The HSV-1 may have initially entered the vagus ganglia through the oral pharynx or other peripheral connecting sites. Upon activation, the virus would travel down the vagal nerve to the potential site of peptic ulcer lesion. HSV-1 itself might cause ulcerative lesion in selected cases of a subset of peptic ulcer diseases by directly infecting the mucosal cells in the stomach and duodenum following virus release from neuroendocrine cells or vagal nerve terminals or both. Alternatively, peptic ulcer might activate latent HSV-1 in vagal ganglia, making replication of HSV-1, a contributing but not an initiating factor of ulcer[13].
The detection percentage of positive HSV-1 was similar between the group of patients with gastric and the group of patients with duodenal ulcer (32.4% and 30.4% respectively), in contrast to previous studies, where the viral DNA is demonstrated only in tissue samples from gastric ulcers[13,14]. The exact cellular localization of HSV-1 DNA could not be identified.
On the other hand, investigating a possible association between H pylori and HSV-1 in pathogenesis of a subset of gastroduodenal ulcers, our data suggest that the PCR HSV-1 positivity is associated with a 5.4-fold increase in negative H pylori detection. Moreover, the patients with ulcer lesions infected with HSV-1 presented a similar prevalence of H pylori infection as the control group, which was significantly lower than that in the HSV-1 negative ulcer cases (P = 0.09). This finding requires further investigation.
Possible interpretations for the increased HSV-1 DNA positive detection rate in H pylori negative ulcers include the following. H pylori negativity is influenced by the viral expression, HSV-1 negativity influences H pylori infection and the virus independently causes some gastroduodenal ulcers.
According to our results, in the subgroup of patients with duodenal ulcers, the risk of H pylori infection was independent from HSV-1 DNA expression (P = 0.634). On the other hand, in the subgroup of patients with gastric ulcer disease, the possibility of H pylori negativity was 18.5-fold higher (P = 0.002). These data are in accordance with those reported by Lohr et al[13].
Additionally, according to our results, there was not any correlation between peptic ulcer disease and age, sex, ulcer site, family history of gastroduodenal ulcers, history of H. labialis, alcohol consumption and NSAIDs use. On the contrary, statistically significant difference (P = 0.019) was observed between patients with peptic ulcers and controls, as far as smoking was concerned.
Our results indicate an involvement of HSV-1 in the pathogenesis of peptic ulcer disease. Although an opportunistic infection with the virus in the ulcer site cannot be excluded, the inverse relationship between HSV-1 detection and H pylori infection indicates a possible implication of this virus in the formation of the ulcer crater, at least in a subgroup of patients. Furthermore, experimental data support this[13,15,16,30,31]. The exact localization of the virus in ulcer tissue cells should be precisely determined in order to clarify whether the lesion is caused by HSV-1 or the virus opportunistically is established, especially in immunocompromised patients[32].
| 1. | Wirth HP. Gastroduodenal ulcer disease: update on pathogenesis. Praxis (Bern 1994). 1995;84:570-580. [PubMed] |
| 2. | Brooks MJ, Maxson CJ, Rubin W. The infectious etiology of peptic ulcer disease. Diagnosis and implications for therapy. Prim Care. 1996;23:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Peura DA. Ulcerogenesis: integrating the roles of Helicobacter pylori and acid secretion in duodenal ulcer. Am J Gastroenterol. 1997;92:8S-13S; discussion 13S-16S. [PubMed] |
| 7. | Bader C, Crumpacker CS, Schnipper LE, Ransil B, Clark JE, Arndt K, Freedberg IM. The natural history of recurrent facial-oral infection with herpes simplex virus. J Infect Dis. 1978;138:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Rand KH, Jacobson DG, Cottrell CR, Koch KL, Guild RT, McGuigan JE. Antibodies to herpes simplex type 1 in patients with active duodenal ulcer. Arch Intern Med. 1983;143:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Kottaridis SD, Mihas TA, Goula I, Mihas AA. Herpes viruses and duodenal ulcer disease. J Med Virol. 1989;29:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Archimandritis A, Markoulatos P, Tjivras M, Alexiou A, Kordossi A, Kordossis T, Fertakis A. Herpes simplex virus types 1 and 2 and cytomegalovirus in peptic ulcer disease and non-ulcer dyspepsia. Hepatogastroenterology. 1992;39:540-541. [PubMed] |
| 11. | Rune SJ, Vestergaard BF. IgA antibodies to herpes simplex virus type 1 in duodenal juice and saliva from patients with peptic ulcer and non-ulcer controls. Scand J Gastroenterol. 1984;19:81-84. [PubMed] |
| 12. | Hari VR, Ananthakrishnan N, Kate V, Badrinath S. Can duodenal ulcer perforation be linked to herpes simplex virus infection? Indian J Gastroenterol. 2004;23:5-7. [PubMed] |
| 13. | Löhr JM, Nelson JA, Oldstone MB. Is herpes simplex virus associated with peptic ulcer disease? J Virol. 1990;64:2168-2174. [PubMed] |
| 14. | Kemker BP, Docherty JJ, De Lucia A, Ruf W, Lewis RD. Herpes simplex virus: a possible etiologic agent in some gastroduodenal ulcer disease. Am Surg. 1992;58:775-778. [PubMed] |
| 15. | Warren KG, Brown SM, Wroblewska Z, Gilden D, Koprowski H, Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978;298:1068-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 177] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Rand KH, Berns KI, Rayfield MA. Recovery of herpes simplex type 1 from the celiac ganglion after renal transplantation. South Med J. 1984;77:403-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | McGeoch DJ, Dalrymple MA, Dolan A, McNab D, Perry LJ, Taylor P, Challberg MD. Structures of herpes simplex virus type 1 genes required for replication of virus DNA. J Virol. 1988;62:444-453. [PubMed] |
| 18. | Marshall BJ, Warren JR, Francis GJ, Langton SR, Goodwin CS, Blincow ED. Rapid urease test in the management of Campylobacter pyloridis-associated gastritis. Am J Gastroenterol. 1987;82:200-210. [PubMed] |
| 19. | Thillainayagam AV, Arvind AS, Cook RS, Harrison IG, Tabaqchali S, Farthing MJ. Diagnostic efficiency of an ultrarapid endoscopy room test for Helicobacter pylori. Gut. 1991;32:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Stevens A. Micro-organisms. Theory and Practice of Histological Techniques, 3rd ed. Edinburg-London: Churchill Livingston 1990; 289-308. |
| 21. | Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J Clin Gastroenterol. 1997;24:2-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 217] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 22. | Sprung DJ, Apter MN. What is the role of Helicobacter pylori in peptic ulcer and gastric cancer outside the big cities? J Clin Gastroenterol. 1998;26:60-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Ciociola AA, McSorley DJ, Turner K, Sykes D, Palmer JB. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Chan HL, Wu JC, Chan FK, Choi CL, Ching JY, Lee YT, Leung WK, Lau JY, Chung SC, Sung JJ. Is non-Helicobacter pylori, non-NSAID peptic ulcer a common cause of upper GI bleeding? A prospective study of 977 patients. Gastrointest Endosc. 2001;53:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Xia HH, Wong BC, Wong KW, Wong SY, Wong WM, Lai KC, Hu WH, Chan CK, Lam SK. Clinical and endoscopic characteristics of non-Helicobacter pylori, non-NSAID duodenal ulcers: a long-term prospective study. Aliment Pharmacol Ther. 2001;15:1875-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Dargan DJ, Subak-Sharpe JH. The effect of triterpenoid compounds on uninfected and herpes simplex virus-infected cells in culture. I. Effect on cell growth, virus particles and virus replication. J Gen Virol. 1985;66:1771-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Poswillo DE, Roberts GJ. Topical carbenoxolone for orofacial herpes simplex infections. Lancet. 1981;2:143-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | van der Spuy S, Levy DW, Levin W. Cimetidine in the treatment of herpesvirus infections. S Afr Med J. 1980;58:112-116. [PubMed] |
| 29. | Wiley CA, Schrier RD, Denaro FJ, Nelson JA, Lampert PW, Oldstone MB. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J Neuropathol Exp Neurol. 1986;45:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 90] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Alexiu O, David S, Cajal N, Gruia M, Gologan R, Nicolescu P. Gastroduodenal ulcer obtained by experimental herpes virus inoculation. Virologie. 1976;27:61-62. [PubMed] |
| 31. | Gesser RM, Valyi-Nagy T, Fraser NW, Altschuler SM. Oral inoculation of SCID mice with an attenuated herpes simplex virus-1 strain causes persistent enteric nervous system infection and gastric ulcers without direct mucosal infection. Lab Invest. 1995;73:880-889. [PubMed] |
| 32. | Howiler W, Goldberg HI. Gastroesophageal involvement in herpes simplex. Gastroenterology. 1976;70:775-778. [PubMed] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK