Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6389
Revised: April 9, 2005
Accepted: April 12, 2005
Published online: October 28, 2005
AIM: To insert the constructed TGF-β1 epitope gene into the el loop of C-terminus of truncated hepatitis B core antigen to increase TGF-β1 antigenicity in its prokaryotic expression system and to identify immunity of the expressed recombinant protein in order to exploit the possibility for obtaining anti- TGF-β1 vaccine.
METHODS: The TGF-β1 encoding epitope gene (the mature TGF-β1 from 78-109 amino acid residues, TGF-β132) was amplified by polymerase chain reaction from the recombinant pGEM-7z/ TGF-β132 vector. The HBcAg gene fragments (encoding HBcAg from 1-71 and 89-144 amino acid residues) were amplified from PYTA1-HBcAg vector. The recombinant vector pGEMEX-1 was used to insert HBcAg1-71, TGF-β132 and HBcAg89-144 into restrictive endonuclease enzyme and ligated with T4 ligase. The fusion gene fragments HBcAg1-71-TGF-β132- HBcAg89-144 were recloned to pET28a(+) and the DNA sequence was confirmed by the dideoxy chain termination method. The recombinant vector pET28a (+)/CTC was transformed and expressed in E. coli BL21 (DE3) under induction of IPTG. After purification with Ni+2-NTA agarose resins, the antigenicity of purified protein was detected by ELISA and Western blot and visualized under electron microscope.
RESULTS: Enzyme digestion analysis and sequencing showed that TGF-β1 epitope gene was inserted into the el loop of C-terminus of truncated hepatitis B core antigen. SDS-PAGE analysis showed that relative molecular mass (Mr) of the expressed product by pET28a (+)/CTC was Mr 24 600.The output of the target recombinant protein was approximately 34.8% of the total bacterial protein, mainly presented in the form of inclusion body. Western blotting and ELISA demonstrated that the fusion protein could combine with anti-TGF-β1 polyclonal IgG but not with anti-HBcAg. The purity of protein was about 90 % and the protein was in the form of self-assembling particles visualized under electron microscope. This fusion protein had good anti-TGF-β1 antigenicity and could be used as anti-TGF-β1 vaccine.
CONCLUSION: A recombinant prokaryotic expression system with high expression efficiency of the target TGF-β1 epitope gene was successfully established. The fusion protein is in the form of self-assembling particles and HBcAg can increase the antigenicity of TGF-β1. The expressed TGF-β1 epitope gene shows good immunogenicity and antigenicity.
- Citation: Guo YH, Hao ZM, Luo JY, Wang JH. Construction of prokaryotic expression system of TGF-β1 epitope gene and identification of recombinant fusion protein immunity. World J Gastroenterol 2005; 11(40): 6389-6394
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6389
Liver fibrosis is commonly resulted from diverse liver injuries. Liver fibrosis can be reversed while liver cirrhosis is irreversible[1]. However, no efficient therapy is available at present for this disease[2]. Searching for a new therapy seems very important. Transforming growth factor (TGF-β), especially TGF-β1, plays a pivotal role in the fibrogenesis of liver fibrosis and cirrhosis. TGF-β1, a major cytokine, can regulate the production, degradation, and accumulation of ECM[3-6]. Over expression or activation of TGF-β1 for a prolonged period of time after tissue damage may induce a fibrosis proliferating response and deposition of ECM, resulting in fibrosis in vital organs[7-9]. Inhibition of TGF-β1 can prevent progression of liver fibrosis and enhance hepatocyte regeneration. Blockage of TGF-β1 may have therapeutic effects on liver fibrosis[10-14].
Anti-cytokine vaccination is a safe and effective pathway to blockage hepatic lesions as long as high antibody levels are maintained[15]. It is difficult to induce high titer antibody since TGF-β1 is a self-protein and B cells are immune silence. Fusion peptide epitope-encoded sequences of HBcAg can enhance TGF-β1 immunogenicity, because HBcAg components help T-cells, and the fusion proteins retain the polymeric or the particulate nature of HBcAg[16].
In this paper, fusion protein sequences carrying the TGF-β1 epitope inserted into the el loop of C-terminus of truncated HBcAg were expressed in E.coli BL21. The fusion protein can form particles. HBcAg has the potential to increase the TGF-β1 antigenicity and immunogenicity. Our results indicate that the fusion protein can be used as an effective anti-TGF-β1 vaccine.
Recombinant vector pGEM-7z/TGF-β132 (TGF-β132 is the encoding gene of mature TGF-β1 79-108 amino acid residues)[17] and vector pET28a (+) were provided by Dr Zhi-Ming Hao (Novagen). Top10 and E.coli BL21strains were provided by Xi’an Huaguang Biology Company. Plasmid pYTA1-HBcAg was constructed by Institute of Virology of Chinese Academy of Preventive Medicine. Rabbit anti-human TGF-β1-IgG was purchased from Santa Cruz. Restriction endonuclease enzymes (EcoRI, Hind III, BamHI, Xhol),TagDNA polymerase and T4 DNA ligase were purchased from Huamei Company and TaKaLa Company. dNTP and isopropyl-β-D-thiogalactopyranoside (IPTG) were obtained from Sigma and oligonucleotide primers were obtained from Shanghai Shenggong Company.
The encoding genes of TGF-β1 epitope (mature TGF-β1 amino acid residues from 79-108) and HBcAg were cloned from amino acid residues 1-71 and 89 - 144.
Oligonucleotide primers were designed to amplify the encoding genes based on GenBank TGF-β132 was cloned from the recombinant plasmid pGEM-7z/ TGF-β132 as the PCR fragment template. The primers have a Xhol site incorporated into the 5’ end and a BamHI site at the 3’ end and their sequences are as follows (5’-3’): CC CTC GAG TGC GTG CCG CAG GCG CTG (forward) and CC GGA TCC GCA GGA GCG CAC GAT CAT (reverse). HBcAg gene was amplified by PCR from the vector pYTA1-HBcAg as the template (constructed by Institute of Virology, Chinese Academy of Preventive Medicine).The primers of HBcAg(1-71) have a EcoRIsite incorporated into the 5’ end and a Xhol site at the 3’ end and their sequences are as follows (5’-3’): GGAATTCATGATTACGCCAAGCTTGGCTGCAGAGTTCCATATG (forward) and CCCTCGAGTCCACCCCAGGTAGCTAGAGTC (reverse) .The primers of HBcAg(89-144) have a BamHIsite incorporated into the 5’ end and a HindIII site at the 3’ end and their sequences are as follows (5’-3’): CGGATCCGGTGGAGTCAACAACACTAATATGGGC (forward) and CCAAGCTTCCGCGGAAGTGTTGATAGGAT (reverse). Total volume per PCR was 100 mL containing 2.5 mol/L each dNTP, 250 nmol/L each of the 2 primers, 15 mol/L MgCl2, 3.0U Taq-plus polymerase, 100 ng DNA template and 1×PCR buffer. The PCR consisted of 30 cycles of denaturation at 94 °C for 60 s, annealing at 52 °C for 30 s, and extension at 72 °C for 30 s. The products were visualized on 10 g/L agarose gel under UV light, pre-stained with ethidium bromide. The PCR products were digested respectively using EcoR/Xhol , XhoI/BamHI, BamHI/HindIII. After digestion with the restriction endonuclease enzymes simultaneously, the expected sizes of target amplification fragments HBcAg1-71, TGF-β132 and HBcAg89-144 was 268, 96 and 177 bp, respectively.
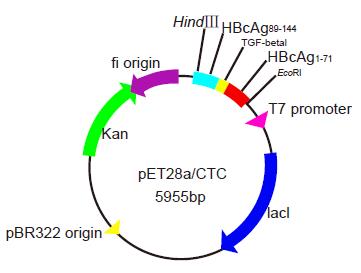
The PCR products were digested respectively using EcoRI/Xhol, XhoI /BamHI, BamHI/HindIII and ligated into the cloning vector pGEM-Teasy. The resulting fragments were cloned into the vector pGEMEX-1. The purified products were cloned into the compatible sites of the vector pGEMEX-1 (Promega, USA) using T4 DNA ligase at a molar ratio of 4:1 at 4 °C overnight. The recombinant plasmid clones pGEMEX-1/ HBcAg1-71- TGF-β132- HBcAg89-144 [HBcAg1-71-TGF-β132-HBcAg89-144 (CTC)] were sequenced using the dideoxy chain termination method. After digestion with the restriction endonuclease enzymes EcoRI and HindIII simultaneously, the fusion gene fragments CTC were cloned into the compatible sites of the prokaryotic expression vector pET-28a(+), downstream of the 6-His tag to facilitate the protein purification using T4 DNA ligase at a molar ratio of 4:1 at 4 °C overnight. The recombinant vector pET28a(+)/CTC was selected and identified by enzyme digestion and sequencing. IPTG at the dose of 1.0 mmol/L could efficiently induce pET28a/CTC in E.coli BL21(DE3) to express the fusion gene composed of 606 base pairs and encode objective polypeptides of 201 amino acid residues(Figure 1).
The single bacterial colony (Top10/pET28a(+)/CTC) was picked and incubated in 2 mL LB containing 100 g/L of Kanamycin, at 300 r/min overnight at 37 °C, then recombinant plasmids were extracted according to the manufacturer’s instructions and identified by PCR and restriction endonuclease enzyme digestion. E.coli BL21 (DE3) strains containing recombinant plasmids were grown until mid-log phase (optical density at 600 nm=0.5-1.0), and then induced to express recombinant fusion protein by adding 1 mmoL/L IPTG for 8 h. Bacteria were harvested by centrifugation at 12 000 g for 2 min, resuspended in protein-buffer and seethed for 5 min. Total protein was electrophoresed on 150 g/L SDS-PAGE gel and stained with Coomassie. The rate of recombinant fusion protein to total protein was deduced by Image Master Band Leader 3.0 software.
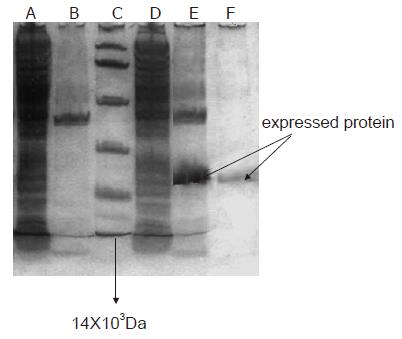
Due to C end of the recombinant fusion antigen with six histidines, Ni2+-NTA agarose resin was used to purify the recombinant fusion antigen. Briefly, 500 ml of cultivated bacterial suspension was prepared, centrifuged, resuspended with the buffer liquid (50 mmoL/L phosphate, 300 mmoL/L NaCl, pH 7.0), and sonicated by ultrasonic wave with the energy of 600 W for 30 min, and ultracentrifuged for 15 min at 10 000 g at 4°C. The sonicated recombinant fusion antigen was purified by Ni2+-NTA agarose resin with abluent (50 mmoL/L phosphate, 300 mmoL/L NaCl, 20 mmoL/L imidazole, pH 7.80) and lavation (50 mmoL/L phosphate, 300 mmoL/L NaCl, 250 mmoL/L imidazole, pH 7.80) respectively, and quantified. pET28a(+)/HBc-TGF-β132 fusion protein was expressed and purified by SDS-15% PAGE. In contrast with the induced product of pET-28a(+) (negative control), a recombinant protein with expected molecular weight of 24.6X103Da was expressed in E. coli BL21 (DE3) as shown by SDS-PAGE.
ELISA The expressed fusion protein was stabilized as antigen at the coated concentration (1:100, 1:200, 1:400, 1:800, 1:1600 dilution) with CBS buffer overnight at 4 °C, and blocked with 100 g/L bovine serum albumin (BSA) for 1 h at room temperature, incubated with the anti-TGF-β1 antibody and HBc antibody as the first antibody respectively for 2 h at room temperature. After a washing, the proteins were detected by incubating in HRP- goat anti-rabbit IgG antibody(1:1000 dilution) (Zhongshan, Beijing) and anti-human IgG (Xi’an Huagguang) as the second antibody respectively for 1 h at room temperature. The first antibodies were replaced by PBS and used as negative control. The result of ELISA for the sample was considered as positive if the A value at 490 nm (A490) was over the mean plus 2.1 SD of the negative samples.
After bacteria BL21/pET-28a(+)/CTC were incubated by adding of 1 mmol./L IPTG for 8 h, 1 ml of cultivated medium was ultracentrifuged, resuspended in protein-buffer and seethed for 5 min. Total protein was electrophoresed on 150 g/L SDS-PAGE gel, and then the proteins of SDS-PAGE-separated (150 g/L acrylamide) were transferred to 0.45 mm pore size PVDF membrane. Following a 30-min washing in tris-saline blotting buffer, antigen-impregated PVDF strips were incubated with anti-TGF-β1 antibody and anti-HBc antibody respectively for 2 h at room temperature. After a washing, the proteins were detected by incubating the strips in alkaline phosphatase-conjugated goat anti-rabbit IgG antibody and anti-human IgG respectively for 1 h at room temperature.
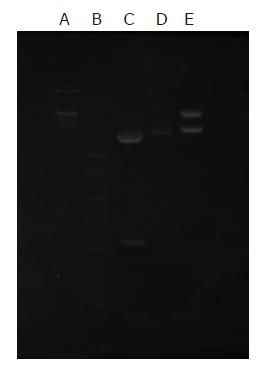
The recombinant vectors pGEMEX-1/ CTC and pET28a(+)/CTC were identified by restriction enzyme digestion in 10 g/L agarose gel electrophoresis. The recombinant vector clones were sequenced using dideoxy chain termination method. The inserted 6xHis- HBcAg1-71-TGF-β132-HBcAg89-144 had the correct open reading frame in the expression vector and correctly sequenced DNA. The nucleotide sequence of the fusion genes was analyzed. Recombinant vector pET-28a(+)/CTC was digested by bi-enzyme digestion with HindIII,EcoRI, then digestive products were visualized on 10 g/L agarose gel (Figure 2). The amplified fused HBcAg1-71-TGF-β132-HBcAg89-144 DNA fragments were confirmed by restriction enzyme digestion and contained the objective gene.
Total protein was electrophoresed on 150 g/L SDS-PAGE gel and stained with Coomassie. Its molecular mass was Mr 24 600 as demonstrated by 150 g/L SDS-PAGE gel analysis. The fusion protein was about 34.8 % of total cellular protein. After purification by Ni2+-NTA agarose resin columniation, the purity of recombinant fusion protein was about 90% (Figure 3).
The antigen-impregated PVDF strips of BL21/pET32a(+)/CTC were recognized by anti-TGF-β1 antibody, showing brown strips corresponding to the site of the recombinant fusion protein. They could not be recognized by HBc antibody, showing no brown strips corresponding to the site of the fusion protein (Figure 4).
Staining was positive in the fusion protein and negative in the negative controls. The expressed fusion protein did not react with anti-HBc polyAb and mAb. The expressed protein reacted with anti-TGF-β1 antibody.
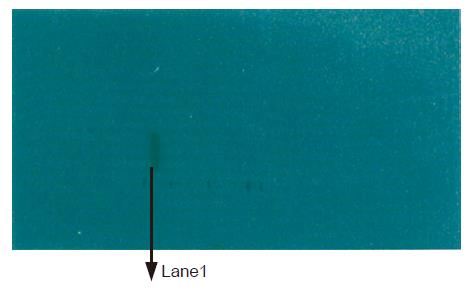
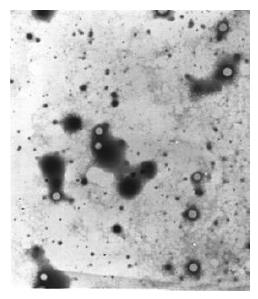
The expressed proteins by the constructs formed core particles observed under electron microscope, which were bigger than HBcAg core particles (Figure 5).
Anti-cytokine vaccination is a safe and effective therapy[19]. Imbalance in cytokine production or cytokine receptor expression and /or dysregulation of cytokine production provides the basis for generating pathological disorders. Cytokine production by effector cells (induced by activation but controlled by negative feedback regulation) does not accumulate in the extracellular compartment under physiologic conditions. The anti-cytokine therapeutic vaccine is to raise antibody levels over the background levels of natural antibodies while enhancing their affinity and neutralizing activity block the accumulation of overproduced cytokines, thereby inhibiting its pathogenic effects. In contrast, the neutralizing Abs do not interfere with cytokine production in normal tissues. Since it has no side effects in normal tissues, high-affinity Abs can be induced safely and effectively.
The TGF-β1 is chosen as an antifibrosis vaccine because it is a key cytokine in hepatic fibrosis and blockade of TGF-β1 prevents liver fibrosis. It is difficult to induce high titer antibody since TGF-β1 is a self-protein. T cells undergo a thymic-negative clonal selection against self-cytokines though anti-cytokine B cells still exist. HBcAg is an attractive candidate for induction of cytokine foreign epitopes because the HBcAg component helps T-cells and the fusion proteins retain the polymeric or the particulate nature of HBcAg. HBcAg behaves as highly immunogenic TI and TD antigens[18,19]. In recent years epitopes of various origin have been inserted into the core antigen of hepatitis B virus (HBV) allowing formation of chimeric HBV core particles. Chimeric core particles carrying the protein can induce protective immunity.
The high immunogenicity of HBcAg depends both on its structure with repetitive spikes on the surface to induce TI responses and on the efficient presentation of HBcAg by B cells to induce TD responses[20]. Fusion of peptide epitope sequences to HBcAg can enhance their immunogenicity. Virus-like particles generated by the heterologous expression of virus structural proteins are able to potentiate the immunogenicity of foreign epitopes presented on their surface. Several foreign epitopes from hepatitis B virus, Papillomavirus, Plasmodium falciparum and hantavirus[21-24] have been introduced successfully into HBcAg capsids, and immunogenicity of the new epitopes has been demonstrated.
Foreign epitope sequence of varying length was inserted into HBcAg in various protein regions, including the N-,C-termini and some internal sites including immunodominant el loop without loss of the self-assembly properties of HBcAg[25].It has been demonstrated that the loop in the main determinant of the core antigen is the most promising insertion site from the immunological point of view. Epitopes inserted there possess higher antigenicity and immunogenicity than anywhere. But not all chimeric proteins are able to be particulated. The ability of chimeric HBcAg to self-assemble is most likely to depend on the physical and chemical properties of the amino acid residues forming the inserted foreign peptide.
The fusion of sequences carryies the epitope of the TGF-β1 (mature encode zone amino acids 78-109)[26] to the el loop of C-terminus of truncated HBcAg, produced in E. coli. BL21 (DE3). The expressed fusion protein is in the form of self-assembling particles observed under electron microscope. The results demonstrate that recombinant fusion protein has a good anti-TGF-β1 antigenicity. Mr 24 600 recombinant fusion protein has HBcAg characteristics and possesses TGF-β1 antigen specialty. The fusion proteins carrying the TGF-β1 antigen-dominant fragment induce antibodies that react effectively with native TGF-β1, suggesting that the recombinant fusion protein can be used as a true vaccine candidate. In the present study, recombinant plasmid was constructed and expressed in BL21(DE3) to explore the possibility for obtaining a vaccine conferring protection against liver fibrosis. We are going to use it in the hepatic fibrosis model as an anti-fibrosis vaccine. Further study is needed to detect its anti-fibrosis effect as a therapeutic vaccine.
This work was supported by Mr.Yang Guangxiao and Wang Quanying of the Xi’an Huaguang Biologic Company.
| 1. | Wang JY, Guo JS, Yang CQ. Expression of exogenous rat collagenase in vitro and in a rat model of liver fibrosis. World J Gastroenterol. 2002;8:901-907. [PubMed] |
| 2. | Murphy F, Arthur M, Iredale J. Developing strategies for liver fibrosis treatment. Expert Opin Investig Drugs. 2002;11:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Si XH, Yang LJ. Extraction and purification of TGFbeta and its effect on the induction of apoptosis of hepatocytes. World J Gastroenterol. 2001;7:527-531. [PubMed] |
| 6. | Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 405] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Sporn MB, Roberts AB. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992;119:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 584] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 8. | Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 889] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2357] [Cited by in RCA: 2405] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 10. | Komesli S, Vivien D, Dutartre P. Chimeric extracellular domain type II transforming growth factor (TGF)-beta receptor fused to the Fc region of human immunoglobulin as a TGF-beta antagonist. Eur J Biochem. 1998;254:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Qi Z, Atsuchi N, Ooshima A, Takeshita A, Ueno H. Blockade of type beta transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc Natl Acad Sci USA. 1999;96:2345-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 234] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 12. | Nakamura T, Sakata R, Ueno T, Sata M, Ueno H. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3' coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265-273. [PubMed] |
| 14. | Ueno H, Sakamoto T, Nakamura T, Qi Z, Astuchi N, Takeshita A, Shimizu K, Ohashi H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther. 2000;11:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 15. | Zagury D, Burny A, Gallo RC. Toward a new generation of vaccines: the anti-cytokine therapeutic vaccines. Proc Natl Acad Sci USA. 2001;98:8024-8029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Schödel F, Peterson D, Hughes J, Milich D. Hepatitis B virus core particles as a vaccine carrier moiety. Int Rev Immunol. 1994;11:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Zhiming Hao, Jinyan Luo, Quanying Wang, Guangxiao Yang, Prokaryotic expression of TGFβ1(78 - 109)-HBV core fusion protein. Xi'an Jiaotong Daxue Xuebao (Medical Sciences). 2003;24:105-108. |
| 18. | Milich DR, Peterson DL, Zheng J, Hughes JL, Wirtz R, Schödel F. The hepatitis nucleocapsid as a vaccine carrier moiety. Ann N Y Acad Sci. 1995;754:187-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986;234:1398-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 297] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 20. | Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, Hughes JL. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 1997;94:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Borisova G, Arya B, Dislers A, Borschukova O, Tsibinogin V, Skrastina D, Eldarov MA, Pumpens P, Skryabin KG, Grens E. Hybrid hepatitis B virus nucleocapsid bearing an immunodominant region from hepatitis B virus surface antigen. J Virol. 1993;67:3696-3701. [PubMed] |
| 22. | Tindle RW, Herd K, Londoño P, Fernando GJ, Chatfield SN, Malcolm K, Dougan G. Chimeric hepatitis B core antigen particles containing B- and Th-epitopes of human papillomavirus type 16 E7 protein induce specific antibody and T-helper responses in immunised mice. Virology. 1994;200:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Schödel F, Wirtz R, Peterson D, Hughes J, Warren R, Sadoff J, Milich D. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. J Exp Med. 1994;180:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Ulrich R, Koletzki D, Lachmann S, Lundkvist A, Zankl A, Kazaks A, Kurth A, Gelderblom HR, Borisova G, Meisel H. New chimaeric hepatitis B virus core particles carrying hantavirus (serotype Puumala) epitopes: immunogenicity and protection against virus challenge. J Biotechnol. 1999;73:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Pumpens P, Borisova GP, Crowther RA, Grens E. Hepatitis B virus core particles as epitope carriers. Intervirology. 1995;38:63-74. [PubMed] |
| 26. | Tahara N, Yasumitsu H, Umeda M. Synthetic peptide-generated monoclonal antibodies to transforming growth factor-beta 1. Hybridoma. 1993;12:441-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK