Published online Oct 28, 2005. doi: 10.3748/wjg.v11.i40.6381
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: October 28, 2005
AIM: To investigate the apoptotic effects of nucleosides on the human hepatoma HepG2.
METHODS: The nucleosides included inosine (I), cytidine (C), uridine (U), thymidine (T), adenosine (A), and guanosine (G). Cells were incubated by the mediums with or without nucleosides at 37 °C in a 50 mL/L CO2 humidified atmosphere.
RESULTS: It was found that the cell viabilities were significantly decreased, when cells were treated with 30 mmol/L I, 30 mmol/L C, 30 mmol/L U, 30 mmol/L T, 0.5 mmol/L A, and 0.5 mmol/L G after 12 h incubation (P<0.05). About the apoptotic phenomenon, the cell percentages of sub-G1 cells were significantly increased in the mediums containing nucleosides such as C, U, T, A, and G (P<0.05). Furthermore, the caspase-3 activity was increased, when the cells were incubated with T (P<0.05). The protein expressions of p53 and p21 showed no difference in each group. To investigate the mechanism of apoptosis induced by nucleosides, it was found that the contents of soluble Fas ligand contents were increased in HepG2 cells following I, U, T, and A treatment (P<0.05). But, TNF-α and cytochrome c were undetectable.
CONCLUSION: Thymidine may induce the apoptosis in HepG2, but the effective dosages and reactive time must be investigated in the future study. However, the apoptosis-inducing abilities of other nucleosides were still unclear in this study.
- Citation: Yang SC, Chiu CL, Huang CC, Chen JR. Apoptosis induced by nucleosides in the human hepatoma HepG2. World J Gastroenterol 2005; 11(40): 6381-6384
- URL: https://www.wjgnet.com/1007-9327/full/v11/i40/6381.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i40.6381
According to the statistics tabulated by the Department of Health, Taiwan in 2003, cancer is the first leading cause of death in Taiwan. Among the various cancers, the morbidity of hepatoma is the highest in Taiwanese. The great medical expenses covering not only drugs but also in providing nutritional supplements for treating or preventing the liver diseases and hepatoma are the heavy burdens of Taiwanese.
Food-derived inducers of apoptosis may be significant as exogenous anti-tumor substances in the control of malignant cell proliferation despite little understanding about their molecular and cellular basis of action. Many healthy foods that are rich in nucleosides have anti-carcinogenic effects, such as the hot water extract of chlorella[1-3]. Current evidence suggests that nucleosides induce cell death by apoptosis[4-6]. Nucleosides that act as signaling molecules are well documented and are mostly released from cells, when cells are under stress, such as anoxia or injury[7-9]. In addition, it has been presumed that a vast majority of tumor promoters are potent inhibitors of apoptosis, and cancer cells did not produce apoptosis-inducing nucleosides[10]. However, little is known about the signaling or the biochemical mechanisms of nucleosides-mediated cell death.
Thus, the purpose of this study was to investigate the apoptosis-inducing ability and the molecular mechanisms of nucleosides in human hepatoma HepG2.
The human hepatoblastoma cell line, HepG2 (HB-8065), was obtained from the American Type Culture Collection. Cells were cultured in MEM supplemented with 5% FBS. Cells were grown in a humidified incubator under 50 mL/L CO2 at 37 °C and medium was changed every 2 d. When the cell status was stable after several passages, the experiments started. Cells were seeded in 100-mm dishes at a density of 1106 cells/well. They were incubated with defined concentrations of nucleosides for 12 h. The cells and media were collected and analyzed after incubation. The nucleosides included inosine (I), cytidine (C), uridine (U), thymidine (T), adenosine (A), and guanosine(G).
The cell viability was determined using CellTiterR96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). The assay is an MTT-based method, which measures the ability of metabolically active cells to convert tetrazolium salt into a cleavage product and its absorbance is recorded at 490 nm[11].
Cells were harvested by 0.25% trypsin release, washed twice with PBS, permeated with 75% ethanol, treated with 3 μL RNase A and finally stained with 1 mL propidium iodide (40 μg/mL final concentration). Distribution of cell-cycle phases with different DNA contents was determined using FACScan flow cytometer (Becton Dickenson, San Jose, CA, USA). Cells that were less intensely stained than G1 cells (sub-G1 cells) in flow cytometric histograms were considered as apoptotic cells.
After treatment for 12 h, cells were collected and lysed with 50 μL cell lysis buffer. Then, the cell lysate was centrifuged and the supernatants were retained to determine the caspase-3 activity with Caspase-3/CPP32 Colorimetric Kit (Biosource International, Camarillo, CA, USA). Furthermore, the soluble Fas ligand contents (sFas-L) in the medium were detected with Human sFas Ligand ELISA kit (Biosource International).
Cell proteins were extracted from cells in a buffer containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 4% protease inhibitors, and 1% phosphatase inhibitors. The cell lysate was rotated at 4 °C for 30 min, centrifuged at 10 000 r/min for 10 min and the precipitates were discarded. Protein contents were determined by the DC Bio-Rad protein assay kit using bovine serum albumin as a standard. The cell proteins were added to an equal volume of 2 sample buffer containing 0.125 mol/L Trizma base, 4% sodium dodecyl sulfate (SDS), 20% glycerol and 10% 2-mercaptoethanol and boiled during 3 min at 100 °C. Then, proteins (100 μg) were separated by 12% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Schleicher & Schuell BioScience, Keene, NH, USA), which was then incubated with primary antibody, such as p53 mouse monoclonal IgG2a (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and F-5 mouse monoclonal IgG2b (Santa Cruz Biotechnology). HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) was used as a secondary antibody. The immunoreactive bands were washed four times in PBS-T (PBS in 0.2% Tween 20), dried and exposed to Kodak film. The relative intensity of the bands was analyzed by densitometry.
All data are expressed as the mean盨D. One-way analysis of variance and Fisher抯 least significant difference test were used to compare the differences of means using the SAS software (v.6.12, SAS Institute, Cary, NC, USA). Statistical significance was assigned at the 0.05 level.
The cell viabilities were significantly decreased when cells were treated with defined concentrations of nucleosides after 12-h incubation (Table 1). The most effective dosages were 30 mmol/L for I, C, U, and T, 0.5 mmol/L for A and G. Thus, these effective concentrations were used in later experiments.
| Treatment | Inosine (%) | Cytidine (%) | Uridine (%) | Thymidine (%) | Adenosine(%) | Guanosine(%) |
| 0 mmol/L | 103.9±3.5a | 101.8±1.6a | 103.5±3.5a | 102.2±1.9a | 101.3±1.2a | 103.1±2.7a |
| 7.5 mmol/L | 94.0±1.2a | 92.0±3.8 a | 100.6±6.6a | 101.5±5.1a | ||
| 15 mmol/L | 69.5±1.8 a | 75.6±1.7 a | 99.1±2.8a | 99.0±0.3a | ||
| 30 mmol/L | 67.7±0.7a | 61.4±5.9a | 86.1±1.3a | 84.5±3.2a | ||
| 0.1 mmol/L | 93.0±5.2a | 86.3±3.5a | ||||
| 0.3 mmol/L | 80.9±2.0a | 89.4±2.1a | ||||
| 0.5 mmol/L | 78.4±1.8a | 86.3±3.3a |
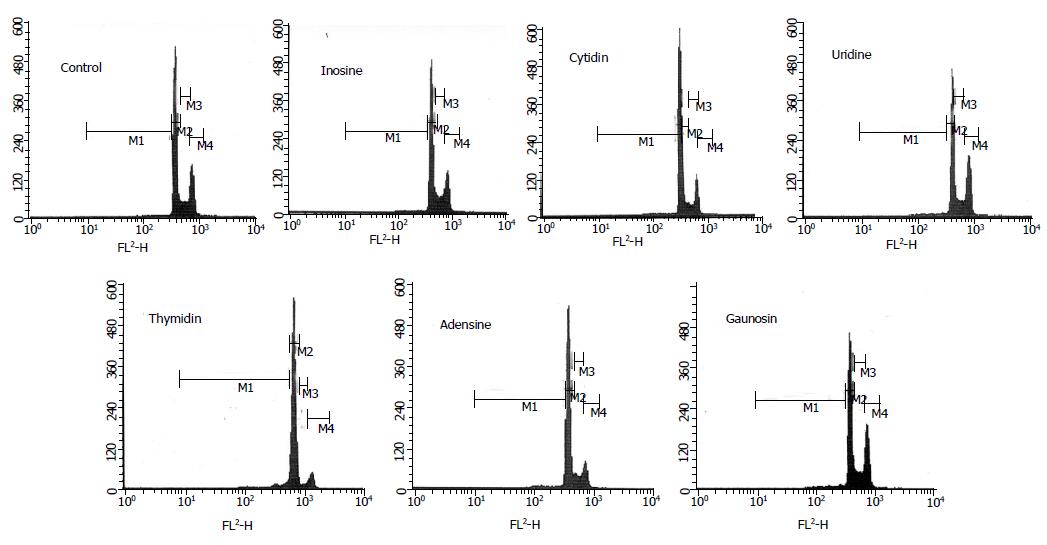
Figure 1 is the example of flow cytometer graph from all results in each group. The quantity of various phase percentages is shown in Table 2. About the apoptotic phenomenon, the cell percentages of sub-G1 phase were significantly increased in the media containing nucleosides such as C, U, T, A, and G (Table 2). In particular, thymidine induced the highest cell percentages of sub-G1 phase among the nucleosides. The cell-cycle progression of I, A, and G groups was similar to that of control group. Moreover, C and U treatments caused an increase in the cell numbers in the G0/G1 phase of the cell cycle. Certainly, the cell numbers of S phase in C and U groups were significantly decreased.
| Treatment | Control | Inosine | Cytidine | Uridine | Thymidine | Adenosine | Gaunosine |
| SubG1 (%) | 2.6±0.8a | 3.2±0.3a | 6.9±0.2a | 6.5±1.5a | 26.8±2.3a | 9.6±1.5a | 5.7±1.2a |
| G0/G1(%) | 67.7±1.1a | 69.2±0.1a | 74.1±1.3a | 84.8±2.6a | 54.4±1.8a | 64.8±1.4a | 68.0±1.9a |
| S (%) | 15.7±0.0a | 18.8±0.3a | 8.5±0.2a | 3.1±0.3a | 12.0±0.2a | 10.6±0.4a | 17.3±0.7a |
| G2/M (%) | 14.0±1.9a | 8.8±0.1a | 10.5±1.0a | 5.7±0.8a | 6.8±0.8a | 15.0±0.9a | 9.0±1.3a |
| Caspase-3 (%)2 | 100±0a | 101±5a | 97±6a | 98±5a | 148±2a | 104±4a | 100±3a |
| sFas-L (pg/mL) | 0.11±0.01a | 0.14±0.01a | 0.08±0.03a | 0.15±0.03a | 0.17±0.02a | 0.15±0.07a | 0.10±0.03a |
Caspase-3 activity was increased when the cells were incubated with T (Table 2). To investigate the mechanism of apoptosis induced by nucleosides, it was found that the contents of sFas-L contents were increased in HepG2 cells following I, U, T, and A treatment (Table 2). But, TNF-α and cytochrome c were undetectable (data not shown).
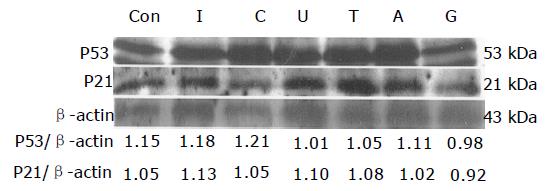
HepG2 is the wild type hepatoma with p53 gene. The expressions of p53 and p21 showed no difference in each group (Figure 1). The densitometric result was also unchanged (Figure 2).
The cytotoxicity of nucleosides at defined concentrations in human hepatoma HepG2 cells was proved in this study. However, it has been reported that 1 mmol/L I, C, U, and T did not inhibit the proliferation of HL-60 and Caco-2 cells[12]. In addition, the inhibition effects were observed after 1 mmol/L A and G treatment[12]. Thus, it was speculated that the different cell type of tumor might lead to completely opposite influences.
The cell percentages of sub-G1 phase were increased after C, U, T, A, and G treatment. However, the variation of sub-G1 phase in C, U, A, and G groups was weaker than other apoptosis-related researches[13,14]. More apoptotic indicators or treatment dosages of nucleosides must be investigated further.
Thymidine increased the caspase-3 activity in HepG2 cells. Moreover, the sFas-L was also increased when cells were cultured with thymidine. However, these effects were not extremely obvious. These results indicated that thymidine might have the potential to induce the apoptosis, but the effective dosage and reactive time must be investigated further.
The cell percentages of sub-G1 phase and caspase-3 activities were still unchanged despite sFas contents were higher after inosine and uridine treatments. Similarly, cytidine did not increase the caspsase-3 activity and sFas-L contents, but raised the cell percentages of sub-G1 phase. Insufficient data indicated the apoptosis-inducing action of inosine, uridine, and cytidine. There are two possibilities, which can explain these results. One is that the apoptosis induced by inosine, uridine, and cytidine might occur later than 12-h incubation. The other is that the cell death induced by inosine, uridine, and cytidine, which showed in MTS assays, was necrosis not apoptosis. Besides, the cell cycle progressions of uridine and cytidine were different from those of other nucleosides. It was observed that uridine and cytidine induced G0/G1 phase arrest in HepG2 cells. This effect may be associated with the growth inhibitory action of uridine and cytidine.
Chow et al. indicated that adenosine induced apoptosis of HL-60 cells by activating the G protein and increasing the cytosolic Ca2+ concentration when adenosine combined with its receptors, P1[15,16]. Moreover, guanosine appears to improve the release of adenosine from cell, and induce cell death via the action of adenosine[17]. But, in this study, the apoptotic-related signals, such as sFas-L content and caspase-3 activity, were not altered in cells after adenosine and guanosine treatments. Thus, the treatment concentration and time point must be reconsidered.
The tumor suppressor p53 is the most commonly mutated gene in human cancers. Cells can respond to the activation of the tumor-suppressor protein p53 by undergoing apoptosis[18,19]. Active p53 is able to stimulate the transcription of a variety of genes including p21, which is a universal inhibitor of the cyclin-dependent kinases (CDK)[20-22]. There were few researches that investigated the relationships between the p53 and nucleosides. In this study, it was found that nucleotides did not alter the expression of p53 and p21 after 12-h incubation with cells.
In conclusion, thymidine might have the potential to induce the apoptosis; however, the apoptosis-induced abilities of other nucleosides were still unclear in this study. It is necessary to recheck the dosage and time of treatment and measure more apoptotic indicators, such as DNA fragmentation and morphological analysis.
| 1. | Tanaka K, Konishi F, Himeno K, Taniguchi K, Nomoto K. Augmentation of antitumor resistance by a strain of unicellular green algae, Chlorella vulgaris. Cancer Immunol Immunother. 1984;17:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Tanaka K, Tomita Y, Tsuruta M, Konishi F, Okuda M, Himeno K, Nomoto K. Oral administration of Chlorella vulgaris augments concomitant antitumor immunity. Immunopharmacol Immunotoxicol. 1990;12:277-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Noda K, Ohno N, Tanaka K, Kamiya N, Okuda M, Yadomae T, Nomoto K, Shoyama Y. A water-soluble antitumor glycoprotein from Chlorella vulgaris. Planta Med. 1996;62:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Van Buren CT, Rudolph F. Dietary nucleotides: a conditional requirement. Nutrition. 1997;13:470-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Chow SC, Kass GE, Orrenius S. Purines and their roles in apoptosis. Neuropharmacology. 1997;36:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Ockner RK. Apoptosis and liver diseases: recent concepts of mechanism and significance. J Gastroenterol Hepatol. 2001;16:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Collis MG, Hourani SM. Adenosine receptor subtypes. Trends Pharmacol Sci. 1993;14:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 278] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Fishman P, Bar-Yehuda S, Ohana G, Pathak S, Wasserman L, Barer F, Multani AS. Adenosine acts as an inhibitor of lymphoma cell growth: a major role for the A3 adenosine receptor. Eur J Cancer. 2000;36:1452-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Wu JM, Lin JS, Xie N, Liang KH. Inhibition of hepatitis B virus by a novel L-nucleoside, beta-L-D4A and related analogues. World J Gastroenterol. 2003;9:1840-1843. [PubMed] |
| 10. | Wright SC, Zhong J, Larrick JW. Inhibition of apoptosis as a mechanism of tumor promotion. FASEB J. 1994;8:654-660. [PubMed] |
| 11. | Kwon KB, Kim EK, Lim JG, Jeong ES, Shin BC, Jeon YS, Kim KS, Seo EA, Ryu DG. Molecular mechanisms of apoptosis induced by Scorpio water extract in human hepatoma HepG2 cells. World J Gastroenterol. 2005;11:943-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Meisel H, Günther S, Martin D, Schlimme E. Apoptosis induced by modified ribonucleosides in human cell culture systems. FEBS Lett. 1998;433:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3574] [Cited by in RCA: 3557] [Article Influence: 122.7] [Reference Citation Analysis (10)] |
| 15. | Chow SC, Kass GE, Orrenius S. Purines and their roles in apoptosis. Neuropharmacology. 1997;36:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Schrier SM, Tilburg EWV, Meulen HVD, Ijzerman AP, Mulder GJ, Nagelkerke . Extracellular adenosine-induced apoptosis in mouse neuroblastoma cells studies on involvement of adenosine receptors and adenosine uptake. Biochem Pharmacol. 2001;61:417-425. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Soussi T. The p53 tumour suppressor gene: a model for molecular epidemiology of human cancer. Mol Med Today. 1996;2:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799-1805. [PubMed] |
| 19. | Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791-1798. [PubMed] |
| 20. | Kagawa S, Fujiwara T, Hizuta A, Yasuda T, Zhang WW, Roth JA, Tanaka N. p53 expression overcomes p21WAF1/CIP1-mediated G1 arrest and induces apoptosis in human cancer cells. Oncogene. 1997;15:1903-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1346] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 22. | Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994;8:2817-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 387] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
Co-first-authors: Suh-Ching Yang and Che-Lin Chiu
Co-correspondents: Suh-Ching Yang
Science Editor Guo SY Language Editor Elsevier HK