Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.573
Revised: November 14, 2003
Accepted: April 13, 2004
Published online: January 28, 2005
AIM: To investigate the protective effect against two immune liver injury models in mice by 2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol hydrochloride and its possible mechanisms in Con A-induced liver damage.
METHODS: Liver tissue or hepatocyte injury was monitored biochemically by measuring alanine aminotransferase (sALT) and aspartate aminotransferase (sAST) activity. Hematoxylin & eosin (HE) staining was used for histopathological examination. To evaluate the role of IFN-γ and IL-4 in the liver injury, serum levels of IFN-γ and IL-4 were determined using commercially available ELISA kit at 12 h after Con A challenge. We also determined FTY 720-induced spleen cell apoptosis by flow cytometry analysis or spleen cell proliferation test.
RESULTS: Different doses of FTY 720 treatment dramatically reduced circulating markers of hepatocyte injury in two kinds of immunological liver injury models. FTY 720 dramatically reduced the elevated serum IFN-γ and IL-4 levels after Con A injection. Effect of spleen cell supernatants treated with Con A or FTY 720 on hepatocytes showed that ALT activities in cultured hepatocyte supernatants in Con A treatment group increased markedly and FTY 720 could reduce this elevated ALT activities in FTY 720 treatment group. FTY 720 dose-dependently increased the percentage of apoptotic cells in T cells and inhibited splenocyte proliferation induced by Con A.
CONCLUSION: Pretreatment with FTY 720 was shown to produce protective effect on the immune liver injury in mice. The possible mechanism of FTY 720 on Con A-induced liver damage is that it could inhibit lymphocyte proliferation and induce lymphocyte apoptosis, resulting in the reduction of IL-4 or IFN-γ release, and subsequently protecting liver from being damaged by Con A.
- Citation: He JH, Zhang HN, Lin ZB. Effect of 2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol hydrochloride (FTY 720) on immune liver injury in mice. World J Gastroenterol 2005; 11(4): 573-576
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/573.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.573
Acute liver injury is a common disease condition? caused by hepatitis virus, alcohol, drugs, etc. Many medicines such as antiviral, immunomodulatory, auxiliary agents have been used to treat acute liver injury, but the therapeutic effects are not so satisfactory[1]. To prevent hepatocyte necrosis in acute stage of liver inflammation, immunosuppressants could be used. It has been reported that cyclosporine A and FK 506 have protective effects on experimentally-induced acute liver injury[2,3]. BCG-pretreatment plus lipopolysaccharide (LPS) or Con A-induced immune liver injury models are two established experimental immune liver injury models commonly used for the study of hepatoprotective medicines[4].
2-amino-2-[2-(4-octylphenyl) ethyl] propane-1,3-diol hydrochloride (FTY 720, C19H33NO2: HCl; molecular weight 343.94 Da), a novel immunosuppressive agent, is a synthetic structural analog of sphingosine related to the drug myriocin(ISP-1), which was isolated from culture filtrates of the ascomycete Isaria sinclairii. Ascomycetes are mycelial forms of Fungi Imperfecti, which are characterized by asexual spore phases. These organisms usually parasitize insects or plants[5]. FTY 720 has been shown to markedly prolong the survival time of rat skin and cardiac allografts[6]. FTY 720 is able to treat the experimental autoimmune thyroiditis in rats, autoimmune diabetes animal model, prostate cancer, and hepatic ischemia-reperfusion injury[7-10]. But, there has been no report about the effect of FTY 720 on immunological liver injury. This study was to evaluate the protective effect of FTY 720 as a new immunosuppressant on experimental immune acute liver injury.
Male mice (albino Swiss) weighing 18-22 g, 6-8 wk old and male Wistar rats (weighing 200-250 g), were all obtained from the Department of Experimental Animals, Peking University Health Science Center, Beijing, China. Animals received humane care according to the criteria outline in the “Guide for the Care and Use of Laboratory Animals” made by the Chinese Academy of Sciences. Mice were maintained under controlled conditions (22 °C, 55% humidity, 12 h day/night cycle) and were fed with a standard laboratory chow until the experiments were completed.
FTY 720 was supplied as dry powders by Hangzhou Sino-Amercan Hua-Dong Pharmaceutical Co. Ltd and dissolved in physiological saline. BCG was purchased from Bioproduct Certification Institute, Beijing. There were also other reagents as follows: LPS (Sigma-Aldrich, St Louis, MO, USA); ALT, AST testing kit (Chemical Reagents Co, Beijing); IFN-γ, IL-4 ELISA kit (Chemicon Co); MTT (Sigma-Aldrich, St Louis, MO, USA) and Con A (Sigma-Aldrich, St Louis, MO, USA).
Effect of FTY 720 on BCG+LPS-induced liver injury The mice were randomly divided into five groups, 10 mice in each. Group 1: normal; group 2: BCG+LPS model control; group 3: BCG+LPS+FTY 720 1 mg/kg; group 4: BCG+LPS+FTY 720 3 mg/kg; group 5: BCG+LPS+FTY 720 6 mg/kg. In the pretreatment groups, three doses of FTY 720 were ig administered into mice once a day. On d 7, the animals in groups 2-5 were injected BCG (3 mg/mouse) intravenously. Another 14 d later, LPS (7.5 μg/mouse) was injected into the mice challenged with BCG. Mice were maintained on their respective drug administration during and after BCG and LPS injection. Twenty-four hours after LPS administration, serum samples from individual mice were obtained from the fundus oculi vein. Liver injury was monitored biochemically by measuring serum alanine aminotransferase (sALT) and aspartate aminotransferase (sAST) activiies.
Effect of FTY 720 on Con A-induced liver injury The mice were randomly divided into five groups, 10 mice in each. Group 1: normal, group 2: Con A 15 mg/kg model control, group 3: Con A 15 mg/kg +FTY 720 1 mg/kg, group 4: Con A 15 mg/kg +FTY 720 3 mg/kg, group 5: Con A 15 mg/kg +FTY 720 6 mg/kg. In the pretreatment groups, three doses of FTY 720 were ig administered into mice once a day, for 10 d. Then animals in groups 2-5 were injected Con A 15 mg/kg intravenously on the tenth day. At 12 h after Con A challenge, serum samples from individual mice were obtained from the fundus oculi vein for the determination of serum ALT and AST activities, as well as serum IFN-γ and IL-4 levels using commercially available ELISA kit. For histopathological evaluation, mice were sacrificed 12 h after Con A challenge. Liver tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of 5 μm thickness were stained with hematoxylin & eosin (HE) for light-microscopic evaluation.
Effects of FTY720 conditioned medium with T lymphocytes (FTY720-T-CM) on isolated hepatocytes Spleen cells from male Wistar rats were isolated by pressing the spleen through a steel grid into RPMI 1640 (GIBCO-BRL, Gaithersburg, MD, USA) medium. Red blood cells were lysed by 2-min incubation with 0.17 mol/L NH4CL at room temperature. Spleen cells were washed twice with RPMI 1640, and 106 cells were incubated in RPMI 1640 containing 10% FCS for 48 h at 37 °C, in an atmosphere containing 50 mL/L CO2 with: 1) medium alone; 2) Con A 5 mg/L; 3) Con A 5 mg/L+FTY 720 31.25 mg/L; 4) Con A 5 mg/L +FTY 720 62.5 mg/L; 5) Con A 5 mg/L +FTY 720 125 mg/L. The supernatants were collected 48 h later and stored at -20 °C.
Hepatocytes were isolated from adult male Wistar rats by collagenase perfusion as described previously[11]. The isolated hepatocytes were suspended in culture medium at 2×106 cells/mL, seeded onto 6-well plastic dishes, and then cultured as monolayers in a CO2 incubator (in a humidified atmosphere of 50 mL/L CO2 in air) at 37 °C. The culture medium used was Dulbecco modified Eagle’s medium (DMEM) (GIBCO-BRL, Gaithersburg, MD, USA) containing 10% FCS, Hepes 15 mmol/L, penicillin 100 units/mL, streptomycin 0.1 g/L, insulin 0.01 g/L, sodium bicarbonate 3.7 g/L. After 12 h, the medium was replaced by fresh DMEM with supernatant of spleen cells (100 μL), and the cells were cultured for 24 h and then the ALT levels in the supernatants were determined.
Effect of FTY720 on spleen lymphocyte proliferation induced by ConA The spleen cell suspension (106 cells/mL) was cultured in a 96-well culture plate in RPMI 1640 containing 10% FCS, Con A and different concentrations of FTY 720. After 68 h, 10 μL of stock MTT at a concentration of 5 mg/mL was added to each well, and the cells were further incubated at 37 °C for 4 h. The supernatant then was removed by centrifugation and 150 μL DMSO was added to each well. Following formazan solubilization, the absorbance of each well was measured using a microculture plate reader at wavelength of 570 nm.
Effect of FTY720 on spleen lymphocyte apoptosis (flow cytometry analysis) Spleen cells were cultured in 24-well plates at a density of 5×106 in 1 mL of medium per well and were given different concentrations of FTY 720 in same volumes. Analysis of apoptosis was performed using annexin V kit (Baosai Co, Beijing). Briefly, one mL of cell suspension (106 cells/mL) was incubated for 3 h under normal control or FTY 720-treated conditions and collected by centrifugation at 1000 r/min at 4 °C for 10 min. Pellets were washed twice with PBS and resuspended in 200 μL binding buffer with 10 μL annexin-V-FITC at room temperature for 10 min. PI (5 μL) was added and analysis was then performed using flow cytometry.
All statistical calculations were performed using SPSS10.0 statistical software; for all analyses, t test and one-way ANOVA were used to test for significance. P<0.05 was considered statistically significant.
Serum ALT and AST activities increased dramatically compared with normal control mice 12 h after BCG+LPS injection. FTY 7206 mg/kg dramatically reduced circulating markers of liver damage as shown in Table 1.
| Group | Number of mice | sALT Karman’s units/dL | sAST Karman’s units/dL |
| Normal | 10 | 19.65±11.87a | 108.00±22.37a |
| BCG (3 mg/mouse) +LPS (7.5 mg/mouse) | 10 | 234.24±143.17 | 254.71±101.45 |
| BCG (3 mg/mouse) +LPS (7.5 mg/mouse) | 10 | 168.05±87.19 | 220.31±54.96 |
| +FTY 720 1 mg/kg BCG (3 mg/mouse)+LPS (7.5mg/mouse)+FTY 720 3 mg/kg | 10 | 162.411±77.43 | 214.21±32.34 |
| BCG (3 mg/mouse)+LPS (7.5 mg/mouse)+FTY 720 6 mg/kg | 10 | 119.411±77.45a | 183.00±46.29a |
Compared with normal control mice, serum ALT and AST activities increased dramatically 12 h after Con A challenge. FTY 720 6 mg/kg dramatically reduced circulating markers of liver injury as shown in Table 2.
Since both IFN-γ and IL-4 play critical roles in Con A-induced liver injury, we investigated the effect of FTY 720 on both serum levels in this model. Different doses of FTY 720 pretreatment dramatically reduced the elevated serum IFN-γ and IL-4 levels after Con A injection as shown in Table 3.
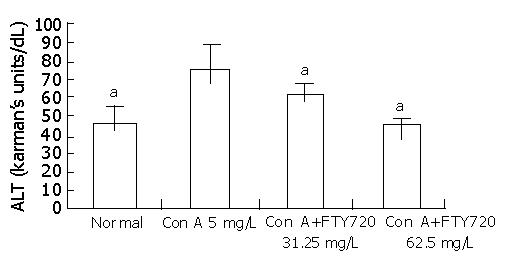
Since the ALT activities were unchanged in supernatants of hepatocyte culture treated directly with Con A or FTY 720 (data not shown), the cultured spleen cell supernatants pretreated with Con A or Con A plus different concentrations of FTY 720 were added into the hepatocyte culture medium for 24 h. The results showed that ALT activities in cultured hepatocytes supernatants in Con A treatment group increased markedly and FTY 720 treatment could reduce the elevated ALT activities as shown in Figure 1.
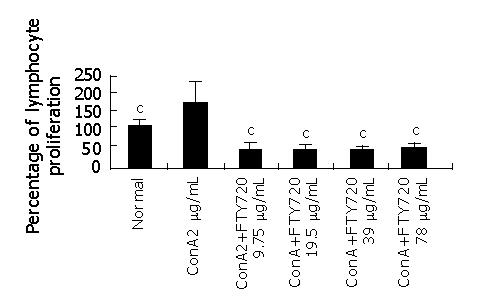
Figure 2 shows that spleen cell proliferation markedly increased when treated with Con A, but a different dose of FTY 720 dramatically reduced the lymphocyte proliferation induced by Con A.
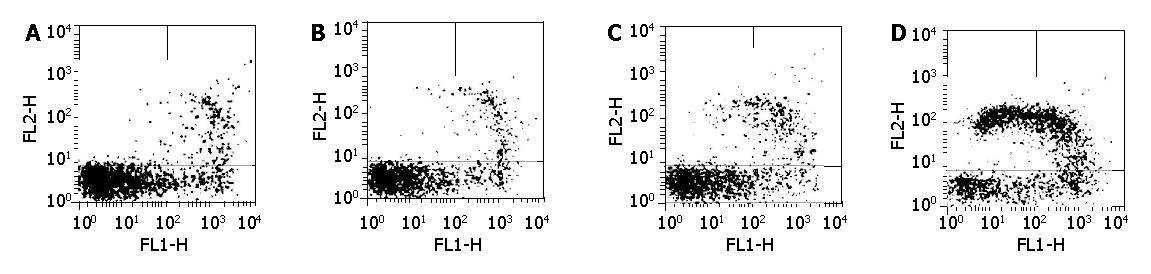
We also investigated the effect of FTY720 on T-lymphocyte apoptosis. A dose-dependent apoptosis was observed in T-lymphocytes as shown in Table 4 and Figure 3. FTY 720 dose-dependently increased the percentage of apoptotic cells in T-cells.
| Group | FTY 720 concentration % | Apoptotic cells |
| Normal | 7.03 | |
| FTY 720 | 1.87 mg/L | 7.55 |
| FTY 720 | 3.75 mg/L | 12.08 |
| FTY 720 | 7.50 mg/L | 25.46 |
FTY 720 is a unique immunosuppressive agent that exerts its activity by inducing apoptosis in lymphocytes[12-14]. We conducted the present study to investigate the effect of FTY 720 on immunological liver injury, as well as its mechanism of action.
Viral hepatitis or autoimmune hepatitis is considered to be involved in the impairment of hepatocytes mainly mediated by T-cell immunity[15]. BCG+LPS or Con A could be used to induce immune liver injury models. T-cell-dependent specific liver injury in mice induced by Con A is a newly estabilished experimental liver injury model. Con A, a kind of lectin, binds to sugar residues on the surface of a wide variety of different cell types and stimulates T lymphocytes and macrophages. Con A facilitates cellular immunity in liver tissue, thereby inducing liver injury. Mice with liver injury induced by Con A are therefore, used as experimental models mediated by cellular immunity. T cell activation and release of several cytokines have been proven to play a critical role in Con A-induced liver injury[16-18]. It has been reported that inflammatory cytokines such as, TNF-α, IFN-γ and IL-4 are elevated in Con A-induced liver injury and inhibition of TNF-α, IFN-γ and IL-4 by antibodies suppresses liver injury[19-21].
In the present study, serum ALT, AST activities, which were the circulating markers of hepatocyte injury, elevated markedly after Con A or BCG+LPS challenge. Pretreatment of FTY 720 could reduce the elevation of serum AST and ALT levels in these two immune liver injury models. These results demonstrate that pretreatment of FTY 720 can elicite liver-protection effect against immune liver damage.
It has been reported that FTY720 prolongs graft survival in various animal models of organ transplantation and other autoimmune diseases such as autoimmune diabetes, autoimmune arthritis because it can induce programmed cell death. In the in vivo study, we found that in Con A-induced liver injury, IFN-γ and IL-4 levels increased significantly as compared with the normal control group, while FTY 720 administration reduced elevated IFN-γ and IL-4 levels. Thus we speculated the liver-protection effect of FTY 720 on Con A-induced liver injury is due to its apoptosis inducing effect on spleen lymphocytes.
We further investigated the effect of FTY 720 on the splenocyte proliferation and apoptosis. The results showed that FTY 720 could inhibit the spleen cell proliferation and promote lymphocyte apoptosis in vitro. Next, we added the supernatant of spleen cell culture treated with FTY 720 to the isolated hepatocyte culture medium. The results demonstrated that the supernatant of spleen cell culture treated with FTY 720 could inhibit the elevated ALT activity induced by the supernatants of spleen cell culture treated only with Con A. These results confirm our previous speculation, that is, the effects of FTY 720 on Con A-induced liver injury were closely associated with spleen lymphocyte apoptosis and proliferation.
In summary, FTY 720 pretreatment is effective on the BCG+LPS and Con A-induced immune liver injuries. The possible mechanism of the liver-protective effect on the Con A-induced liver injury is related to lymphocyte apoptosis and proliferation. Studies are underway to clarify the detailed mechanism.
| 1. | Luo KX. Hepatitis B Basic Biology and Clinical Science. 2nd ed,Beijing: People’s Health Publishing House 2001; 381-444. |
| 2. | Zhang XL, Quan QZ, Sun ZQ, Wang YJ, Jiang XL, Wang D, Li WB. Protective effects of cyclosporine A on T-cell dependent ConA-induced liver injury in Kunming mice. World J Gastroenterol. 2001;7:569-571. [PubMed] |
| 3. | Kaibori M, Inoue T, Tu W, Oda M, Kwon AH, Kamiyama Y, Okumura T. FK506, but not cyclosporin A, prevents mitochondrial dysfunction during hypoxia in rat hepatocytes. Life Sci. 2001;69:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Hershkoviz R, Bruck R, Aeed H, Shirin H, Halpern Z. Treatment of concanavalin A-induced hepatitis in mice with low molecular weight heparin. J Hepatol. 1999;31:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Adachi K, Kohara T, Nakano N, Arita M, Chiba K, Mashin T, Sassaki S, Fujita T. Design, Synthesis and structure-activity relationship of 2-substituted-2-amino-1, 2-propanediols: discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem Lett. 1995;5:853-856. [RCA] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 244] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Sasayama S. FTY720, a new immunosuppressant, promotes long-term graft survival and inhibits the progression of graft coronary artery disease in a murine model of cardiac transplantation. Circulation. 1999;100:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Hozumi Y, Kobayashi E, Miyata M, Fujimura A. Immunotherapy for experimental rat autoimmune thyroiditis using a novel immunosuppressant, FTY720. Life Sci. 1999;65:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Yan H, Suzuki K, Li XK, Amemiya H, Suzuki S, Hiromitsu K. Immunosuppressive effect of FTY 720 on autoimmune diabetes models. Transplant Proc. 1998;30:3436-3437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Permpongkosol S, Wang JD, Takahara S, Matsumiya K, Nonomura N, Nishimura K, Tsujimura A, Kongkanand A, Okuyama A. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer. 2002;98:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Anselmo D, Amersi FF, Shen XD, Gao F, Katori M, Ke B, Lassman C, Coito AJ, Brinkmann V, Busuttil RW. FTY720: a novel approach to the treatment of hepatic ischemia-reperfusion injury. Transplant Proc. 2002;34:1467-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kanemaki T, Kitade H, Hiramatsu Y, Kamiyama Y, Okumura T. Stimulation of glycogen degradation by prostaglandin E2 in primary cultured rat hepatocytes. Prostaglandins. 1993;45:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Suzuki S, Li XK, Enosawa S, Shinomiya T. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology. 1996;89:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Nagahara Y, Enosawa S, Ikekita M, Suzuki S, Shinomiya T. Evidence that FTY720 induces T cell apoptosis in vivo. Immunopharmacology. 2000;48:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Fujino M, Li XK, Guo L, Kitazawa Y, Funeshima N, Fukuda S, Kimura H, Miyashita T, Okuyama T, Amano T. T-cell apoptosis triggered by FTY720 via mitochondrial pathway. Transplant Proc. 2001;33:3084-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 176] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Leist M, Wendel A. A novel mechanism of murine hepatocyte death inducible by concanavalin A. J Hepatol. 1996;25:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, Muto Y, Moriwaki H. Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol. 1999;11:1491-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190-198. [PubMed] |
| 19. | Cao Q, Batey R, Pang G, Russell A, Clancy R. IL-6, IFN-gamma and TNF-alpha production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol. 1998;76:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (3)] |
| 20. | Nishikage T, Seki S, Toyabe S, Abo T, Kagata Y, Iwai T, Hiraide H. Inhibition of concanavalin A-induced hepatic injury of mice by bacterial lipopolysaccharide via the induction of IL-6 and the subsequent reduction of IL-4: the cytokine milieu of concanavalin A hepatitis. J Hepatol. 1999;31:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Kato M, Ikeda N, Matsushita E, Kaneko S, Kobayashi K. Involvement of IL-10, an anti-inflammatory cytokine in murine liver injury induced by Concanavalin A. Hepatol Res. 2001;20:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Edited by Zhu LH and Wang XL Proofread by Ma JY