Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6104
Revised: May 15, 2005
Accepted: May 20, 2005
Published online: October 21, 2005
AIM: To assess whether the effectiveness of a combination of transarterial chemoembolization (TACE) and percutaneous ethanol injection (PEI) in the treatment of unresectable hepatocellular carcinoma (HCC) is superior to TACE alone a randomized controlled trial was performed.
METHODS: The effect of combination therapy on long-term survival rates and duration of hospitalization was evaluated in 52 previously untreated HCCs, randomly allocated to TACE-PEI (27 pts) or TACE alone (25 pts).
RESULTS: The cumulative survival rate of the TACE group was 75.8% at 6 mo, 62.9% at 12 mo, and 18.0% at 24 mo and of the TACE-PEI group 76.9%, 61.5%, and 38.7%, respectively. Comparison of overall survival in both groups showed no statistically significant difference. Regarding the patients with HCCs Okuda stage I (n = 26), the median survival of the TACE-PEI group was significantly longer (>24 mo, median not yet reached in the study period) compared to the TACE group (18.4 mo [range 11.6-21.7 mo]; p = 0.04). TACE-PEI reduced the relative risk for mortality to 0.4 (95%CI 0.15-0.96) compared to patients who received TACE alone. Median survival in patients with HCCs Okuda stage II or III was 5.0 mo in the TACE group (1.7 mo-not defined) compared to 10.4 mo in the TACE-PEI group.
CONCLUSION: The combination TACE-PEI improved survival time compared to TACE alone. Our study revealed a statistically significant improved survival in HCCs Okuda stage I. Side effects were minor and the combination therapy did not prolong duration of hospitalization considerably.
- Citation: Becker G, Soezgen T, Olschewski M, Laubenberger J, Blum HE, Allgaier HP. Combined TACE and PEI for palliative treatment of unresectable hepatocellular carcinoma. World J Gastroenterol 2005; 11(39): 6104-6109
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6104
Hepatocellular carcinoma (HCC) is one of the major malignancies worldwide with the highest incidence in Asia and sub-Saharan Africa[1]. Potentially curative therapeutic strategies such as orthotopic liver transplantation and hepatic resection are possible only in a minority of patients[2-7]. Due to tumor size and multicentricity, comorbidity and advanced or decompensated liver cirrhosis 70-80% of HCCs are inoperable at the time of diagnosis[8]. Because HCCs are chemo- and radio-resistant, systemic chemotherapy and radiation therapy are not options for the treatment[9]. Therefore, in most HCC patients, percutaneous ethanol injection (PEI) or thermal ablation, e.g., radiofrequency thermal ablation, and transarterial chemoembolization (TACE) are palliative treatment options[5,10-14].
Ultrasound guided PEI has been shown to be highly effective in the treatment of small HCCs[15-17]. However, PEI treatment was shown to be of limited efficacy in patients with large HCC (>5 cm) because ethanol diffusion within the tumor mass is incomplete and residual viable neoplastic tissue often persists in the periphery of the tumor lesion.
Advanced HCCs have been widely treated with TACE, using various combinations of chemotherapeutic drugs and embolic agents. The standard protocol adopted in many institutions includes repeated intra-arterial injections of a chemotherapeutic agent such as cisplatin, doxorubicin, or mitomycin C mixed with lipiodol. Lipiodol is added because of its relative selective uptake in HCCs and its antitumor effect[18]. The injection of chemotherapeutic oil emulsion is followed by embolization of feeding arteries with small gelatin-sponge particles.
A potential benefit of combining TACE with PEI in the treatment of HCC was first suggested by Tanaka et al[19]. Adding PEI after TACE has been expected to achieve complete necrosis of the main tumors and to alter the texture of the tumor parenchyma, so that a large volume of ethanol can be administered to penetrate entire tumor lesions resulting in a complete necrosis[20]. In a previous study, we prospectively analyzed the clinical factors determining the prognosis of 132 inoperable HCC patients treated at our institution. Multivariate analysis revealed that patients treated with TACE-PEI had a significantly longer survival than patients treated with PEI or TACE alone[15]. These observations prompted us to do a multicenter randomized controlled trial evaluating the effect of combination therapy with TACE-PEI vs TACE on long-term survival rates, duration of hospitalization and quality of life.
The study was performed as a multicenter randomized controlled trial comparing treatment with TACE and TACE-PEI. The mean 3-year-survival rate in patients treated with TACE is about 30%[18,21-24]. To demonstrate an improvement of the 3-year-survival from 30% to 40% with a statistical power of 80%, it was calculated that 308 patients in each group were needed (group 1 = TACE treatment, group 2 = treatment with TACE in combination with PEI). As a period of recruitment, 36 mo were calculated, and 24 mo were planned as follow-up period.
Participants for the trial were recruited from five centers listed in the Appendix. Patients included in the study fulfilled the following criteria: Untreated patients with HCC, confirmed histologically or diagnosed by significantly elevated a-fetoprotein (AFP) levels (>250 ng/mL) and imaging by CT, MRI, ultrasound, or angiography. No indication for surgery or any local treatment, no evidence for extrahepatic metastases, assessed by clinical and chest X-ray examinations. In patients with clinically suspected bone metastases, a bone scan was performed. There was no age limit. Exclusion criteria were: Second malignancy, any local, or systemic pretreatment, extrahepatic metastases, Child-Pugh C score, complete portal vein thrombosis (main trunk or both branches), serum creatinine >1.5 mg/dL, severe- and therapy-resistant hepatic encephalopathy, contraindications for peripheral artery catheterization. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the University Hospital Freiburg. All participants provided written informed consent. By external blockwise randomization centrally performed by fax, patients were assigned to treatment with TACE only or treatment with TACE-PEI.
TACE A diagnostic angiography was performed in all patients. For TACE, the tip of the catheter was placed in the left or right hepatic artery. Under fluoroscopic guidance, 5-10 mL of a solution containing 10 mg mitomycin C (Medac, Hamburg, Germany) in 10 mL iodized oil was injected into the artery feeding the lesion(s). The mitomycin/oil mixture was prepared immediately before injection by vigorous mixing for a few minutes. Embolization was performed with gelatin-sponge particles (Gelfoam; Upjohn, Kalamazoo, MI, USA). In case of unilateral portal vein thrombosis and contralateral localization of the HCC(s), chemoperfusion without embolization (TAC) of the corresponding lobe was performed. At the end of the procedure, the extent of vascular occlusion was demonstrated angiographically. The patients stayed in general, 1 d in the hospital for observation. Two weeks after TACE, a CT scan was performed. The results were interpreted as follows: (1) tumor necrosis: hypo-attenuated and contrast medium-non-enhanced lesion(s) in the early arterial phase; (2) residual tumor: contrast medium-enhanced lesion(s).
The procedure was repeated every 6-10 wk until one of the following end points was reached: (1) no viable tumor cells detectable by CT or biopsy; (2) contraindications for the procedure developed; (3) patient’s death. In patients who developed thrombosis of the main portal trunk, TACE was performed only in cases of compensated cirrhosis Child-Pugh A score and adequate collateral circulation around the thrombosis (cavernous transformation with prograde intrahepatic portal perfusion on ultrasound Doppler flow examination). Patients not qualifying for TACE received best supportive care, including treatment of complications of liver cirrhosis and pain relief.
Combination of TACE-PEI Patients assigned to TACE-PEI additionally received PEI treatment for all visible lesions, 10 d after TACE. PEI was performed under real-time ultrasound guidance. A 3-MHz convex probe (Ultramark 4; ATL, Solingen, Germany) with a lateral guide attachment or a 3.5-MHz convex probe (EUB 525; Hitachi, Wiesbaden, Germany) with an incorporated guide was used. Two needle types were used: a 15-cm, 20-gauge spinal needle (Becton Dickinson, Franklin Lakes, NJ, USA) or a 20-cm, 20-gauge needle with a closed conical tip and side holes at the tip over a length of 1 or 2 cm (Pflugbeil, Ottobrunn, Germany). Ethanol (96%, 1-10 mL) was slowly injected. The PEI result was photodocumented. In general, PEI treatment involved 6-12 injections per lesion 1-6 d apart. After PEI treatment, patients regularly stayed in general 1 day in the hospital for observation. The procedure was repeated after every TACE, until one of the end points described above was reached.
As primary end point of the study, death of any cause was chosen. Secondary end points were tumor response, quality of life, side effects, and duration of hospitalization because of treatment or side effects.
Follow-up period was 24 mo. Patients were seen on an outpatient basis 1 mo after initiation of the therapy, later on for every 3 mo. Routine follow-up included clinical examination, determination of AFP, and abdominal ultrasound. Abdominal CT or MRI was performed after 1, 3, and 6 mo, and then for every 6 mo. If HCC recurrence was suspected based on ultrasonography or AFP elevation, CT or MRI was performed earlier. Chest X-rays were done after 6, 12, and 24 mo. Quality of life evaluation (EORTC QLQ 30) was performed at inclusion in the study and then after 6, 12, and 24 mo. The therapeutic effect was assessed by CT or MRI every 3 mo according to WHO criteria[25]. A complete response was defined as disappearance of all HCC lesions documented by imaging analyses (ultrasound, CT, or MRI), and confirmation 4 or more weeks later. A partial response was defined as more than 50% reduction of the tumor size based on the measurement of the two longest perpendicular diameters of the tumor.
Statistical analyses
Study design was a multicenter randomized controlled trial to compare therapeutic effect of TACE and TACE-PEI.
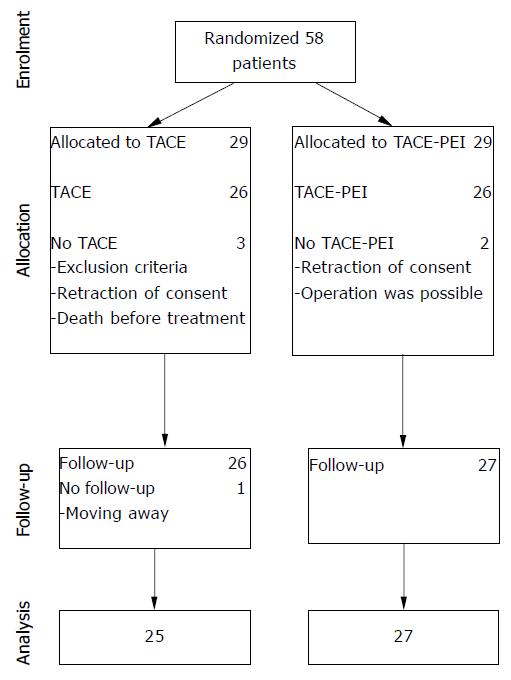
Between January 1997 and August 2001, 58 patients were recruited for the study. Recruitment was stopped after 56 mo. Follow-up period of 24 mo was planned.
Comparison between groups was made on an intention-to-treat basis. Survival time for the TACE- and TACE-PEI group was assessed by the Kaplan-Maier method and log-rank test for univariate analysis. Therapeutic effect was estimated by Cox’s proportional hazards regression model for multivariate analysis. All significance tests were two-sided and P<0.05 was considered statistically significant. Data processing and analysis were performed using the statistical analysis system (SAS Institute, Cary, NC, USA).
From January 1997 to August 2001, 58 patients were enrolled in the study and were randomly assigned to TACE (n = 29) or to TACE-PEI (n = 29). Five patients were excluded because of complete portal vein thrombosis (1 patient), operability (1 patient), death (1 patient) or retraction of consent (2 patients). The remaining 53 patients (26 TACE and 27 TACE-PEI) received allocated intervention. One patient in the TACE group was lost to follow-up after moving. Thus, the data of 52 patients could be analyzed (Figure 1).
Patient characteristics showed no statistically significant differences between the two treatment groups (Table 1).
| TACE | TACE-PEI | |
| Patients (n) | 25 | 27 |
| ean age yr (range) | 63.6 (48-79) | 64 (47-76) |
| M:F (%) | 84:16 | 74.1:25.9 |
| Cirrhosis/no cirrhosis | 22/3 | 23/4 |
| Etiology of cirrhosis (n/%) | ||
| Alcohol | 12 (55) | 13 (57) |
| Hepatitis B or C | 7 (32) | 7 (30) |
| Hemochromatosis | 2 (9) | 1 (4) |
| Unknown | 1 (4) | 2 (9) |
| Liver function (n/%) | ||
| Child Pugh A | 22 (88) | 17 (63) |
| Child Pugh B | 3 (12) | 10 (37) |
| Child Pugh C | 0 | 0 |
| Tumor stage (n) | ||
| Okuda I | 19 | 17 |
| Okuda II | 6 | 9 |
| Okuda III | 0 | 1 |
| AFP | ||
| <100 ng/mL | 14 | 15 |
| ≥100 ng/mL | 11 | 12 |
| Portal vein thrombosis (partial or complete) | 8 | 6 |
| Number of tumor lesions | ||
| 1 | 9 | 13 |
| 2 | 6 | 5 |
| 3 | 5 | 2 |
| >3 | 4 | 5 |
| Diffuse | 0 | 2 |
| Not documented | 1 | 0 |
| Largest diameter >5 cm (US) | 17 | 17 |
At the time of final analysis (median follow-up time 32 mo [range 5-57 mo]), 18 patients of the TACE group and 17 patients of the TACE-PEI group had died. Median survival time was 18.4 mo (range 5.8-22.0 mo) of the TACE group as compared to 15.3 mo (range 9.2-49.0 mo) of the TACE-PEI group.
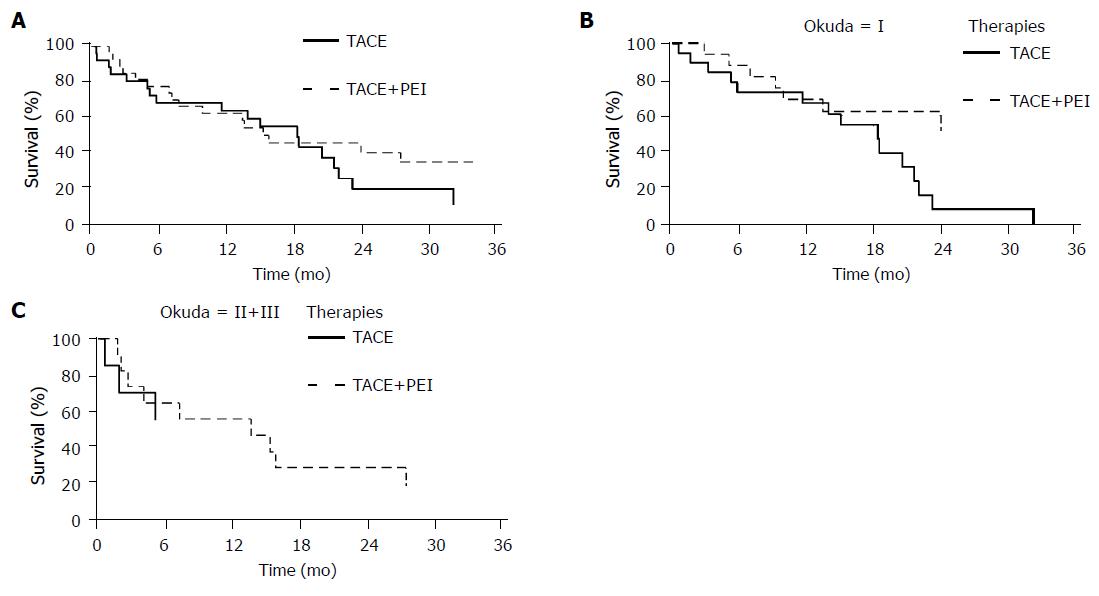
The cumulative survival rate of the TACE group was 75.8% at 6 mo, 62.9% at 12 mo and 18.0% at 24 mo and of the TACE-PEI group 76.9%, 61.5%, and 38.7%, respectively (Figure 2A). Comparison of overall survival in both groups showed no statistically significant difference.
Subgroup analysis of patients with HCC Okuda stage I (n = 26) showed a significant longer survival of the TACE-PEI group (>24 mo, median not yet reached in the study period) as compared to the TACE group (18.4 mo [range 11.6-21.7 mo]; P = 0.04; Figure 2B). TACE-PEI treatment reduced the relative risk for mortality to 0.4 (95%CI 0.15-0.96) compared to patients receiving TACE only.
Median survival of patients with HCCs Okuda stage II or III was 5.0 mo in the TACE group (1.7 mo-not defined) compared to 10.4 mo in the TACE-PEI group (Figure 2C). This difference was not statistically significant (P = 0.39).
Figure 2 A: Overall survival according to treatment; B: Survival of patients with HCCs Okuda stage I according to treatment; C: Survival of patients with HCCs Okuda stage II or III according to treatment.
Four patients in the TACE and five patients in the TACE-PEI group showed stable disease, respectively. The remaining 18 patients in the TACE and 20 patients in the combination group had a progressive disease, respectively (Table 2). In both groups, no major treatment-related complication occurred. The most common side effects of TACE were fever, abdominal pain, and elevation of total bilirubin levels. These side effects were transient and disappeared within a week. Side effects of PEI were spontaneously remitting mild local pain in all patients and mild transient fever in most patients.
| TACE | TACE+PEI | P (χ²-test) | |
| Patients (n) | 22 | 25 | |
| Complete response | - | - | - |
| Partial response | - | - | - |
| Stable disease | 4 | 5 | NS |
| Progressive disease | 18 | 20 | NS |
For all the 52 study patients, we recorded in-patient stays and out-patient visits during follow-up and discriminated between (1) treatment-related hospital stays/visits including adverse effects or complications and (2) hospital stays/visits because of other reasons (Table 3). As expected, patients treated with TACE-PEI had on average slightly longer hospital stays. Overall, however, there was no statistically significant difference between both treatment groups with respect to in-patient days and out-patient visits, respectively.
| TACE | TACE-PEI | P (Wilcoxon rank test) | |
| IST (d) | 33 | 44.67 | 0.46 |
| OST (d) | 2.72 | 3.07 | 0.75 |
| ISO (d) | 9.16 | 5.74 | 0.91 |
To evaluate quality of life, we used a validated self-rating questionnaire (EORTC QLQ 30). According to the design of the study, patients were expected to complete the questionnaire at enrolment into the study and at mo 6, 12, and 24. Unfortunately, only 17 completely filled out questionnaires were obtained. Most patients suffered from poor and worsening physical condition, including hepatic encephalopathy, so that only a minority of patients was able to complete the self-rating questionnaire. Therefore, we could not statistically analyze quality of life.
In a previous study, we prospectively analyzed the clinical factors determining the prognosis of 132 patients with inoperable HCCs who were treated with TACE, PEI, the combination of both, or best supportive care[15]. Multivariate analysis revealed that patients treated with a combination of TACE and PEI had a significantly better survival than patients receiving either PEI or TACE only. These observations prompted us to undertake a multicenter randomized controlled trial evaluating the effect of combination therapy with TACE-PEI on long-term survival.
The purpose of the combination is to overcome the shortcomings of both therapies and to get improved therapeutic results by combined effects. TACE is one of the most common therapies in patients with unresectable HCC. This regional therapy combines targeted chemotherapy and arterial embolization in order to induce both selective ischemic and chemotherapeutic effects on HCC. Although survival benefit has been reported after TACE[5] it is difficult to achieve complete necrosis of the target tumor by TACE alone, because HCC often has intercapsular or extracapsular invasion and viable tumor cells remain after TACE[26]. PEI is also widely used and is an effective means of treating unresectable HCC. The method is effective in cases of capsular invasion. The injection of absolute alcohol causes cellular dehydration, coagulation necrosis, and vascular thrombosis within the treated tumor[27]. But several factors limit the therapeutic effect of PEI, such as tumor consistency, intratumoral septa, tumoral capsule, and tumor vascula-rization[28]. The rationale for combination of TACE and PEI relies on the fact that after TACE tumor consistency is markedly decreased and intratumoral septa are usually disrupted providing enhanced ethanol diffusion within the tumor. Therefore, combined therapy with TACE-PEI has been expected to improve survival rates of patients with advanced HCC.
In our randomized controlled multicenter trial comparing TACE and TACE-PEI, we observed no difference in overall survival. However, subgroup analysis based on HCC Okuda stage revealed that TACE-PEI results in statistically significant improved survival in patients with HCC Okuda stage I.
Similar to our findings, a recently published retrospective analysis based on medical records showed that patients receiving TACE alone had a median survival rate of 16 mo, whereas the median survival rate in patients receiving TACE followed by PEI was 24 mo[29].
The prospective randomized trials by Kamada et al[30] and by Bartolozzi et al[31] also showed improved survival for patients treated with TACE-PEI compared to TACE alone. Because of the small number of patients included in these studies, the difference did not reach statistical significance. Further, Tanaka et al[32] Koda et al[33] and Yamamoto et al[20] showed a significant improved survival for patients treated with TACE-PEI as compared to TACE alone (Table 4).
| Becker et al | Kamada [26] | Bartolozzi [27] | Tanaka [28] | Koda [29] | Yamamoto [20] | |
| Number of patients | ||||||
| 52 | 69 | 53 | 43 | 36 | 100 | |
| Cause of cirrhosis | ||||||
| Hepatitis:alcohol | 14:25 | 65 | 5:48 | No data | No data | 100 |
| 1-yr survival in % of patients | ||||||
| TACE+PEI | 62 | 90 | 100 | 100 | 1001 | 95 |
| TACE | 63 | 86 | 92.6 | 68 | 751 | 92.5 |
| 2-yr survival in % of patients | ||||||
| TACE+PEI | 39 | 3-yr:65 | 86.7 | 85 | 822 | 72.5 |
| TACE | 18 | 3-yr:44 | 69.7 | 37 | 502 | 57.5 |
| P | NS (0.371) | NS | NS | <0.001 | <0.05 | <0.05 |
Even though these studies included patients with Child-Pugh C score liver cirrhosis[20,32,33] mean survival time in our study, which excluded Child-Pugh C score patients, was shorter. This may be due to the following facts: (1) We only excluded patients with complete portal vein thrombosis (main trunk or both branches) whereas others excluded patients with any evidence of (partial) thrombosis[30-33]; that is predicting poor survival[15,20]. (2) Liver disease in our study was caused in about 50% by alcohol, whereas in all other studies most patients had chronic hepatitis B or C (Table 4). Our findings, therefore, may suggest that combination therapy with TACE-PEI may be less effective in patients with alcohol-induced than in patients with HBV- or HCV-induced cirrhosis. (3) Tanaka et al[32] Kodaet al[33] and Yamamoto et al[20] used doxorubicin, whereas in our study mitomycin C was used. Advantages for anthracyclin-based regimes were advocated in one randomized controlled trial[34] but a recently published meta-analysis showed no good evidence for best chemotherapeutic agent[5].
The primary end point of our study was survival time. Secondary end point was the duration of hospitalization and/or the number of out-patient visits. As expected, patients treated with TACE-PEI had on average slightly longer hospital stays than patients with TACE only. There was no statistically significant difference between both treatment groups, however. This aspect is important not only with respect to financial costs, but also with respect to patient’s quality of life.
In conclusion, the combination of TACE-PEI compared with TACE alone prolongs survival in patients at Okuda stage I. Side effects were mild and combined therapy with TACE-PEI did not prolong duration of hospitalization or the number of out-patient visits considerably.
| 1. | Wanebo HJ, Falkson G, Order SE. Cancer of the hepatobiliary system. ): Cancer Principles and Practice of Oncology (3rd ed.), Philadelphia Lippincott 1989; 836-874. |
| 2. | Andriulli A, de Sio I, Solmi L, De Carlis L, Troisi R, Grasso A, Festa V, Caturelli E, Giacomoni A, Del Vecchio Blanco C. Survival of cirrhotic patients with early hepatocellular carcinoma treated by percutaneous ethanol injection or liver transplantation. Liver Transpl. 2004;10:1355-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bruix J. Treatment of hepatocellular carcinoma. Hepatology. 1997;25:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S277-S282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3294] [Article Influence: 143.2] [Reference Citation Analysis (1)] |
| 6. | Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127:S261-S267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 9. | Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies? J Clin Gastroenterol. 2001;32:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179-S188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 398] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 11. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 921] [Article Influence: 34.1] [Reference Citation Analysis (2)] |
| 12. | Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159-S166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 14. | Tanaka K, Nakamura S, Numata K, Kondo M, Morita K, Kitamura T, Saito S, Kiba T, Okazaki H, Sekihara H. The long term efficacy of combined transcatheter arterial embolization and percutaneous ethanol injection in the treatment of patients with large hepatocellular carcinoma and cirrhosis. Cancer. 1998;82:78-85. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Allgaier HP, Deibert P, Olschewski M, Spamer C, Blum U, Gerok W, Blum HE. Survival benefit of patients with inoperable hepatocellular carcinoma treated by a combination of transarterial chemoembolization and percutaneous ethanolinjection - a single center analysis including 132 patients. Int J Cancer. 1998;79:601-605. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Seki T, Nonaka T, Kubota Y, Mizuno T, Sameshima Y. Ultrasonically guided percutaneous ethanol injection therapy for hepatocellular carcinoma. Am J Gastroenterol. 1989;84:1400-1407. [PubMed] |
| 17. | Shiina S, Tagawa K, Unuma T, Fujino H, Uta Y, Niwa Y, Hata Y, Komatsu Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy of hepatocellular carcinoma: analysis of 77 patients. AJR Am J Roentgenol. 1990;155:1221-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mondazzi L, Bottelli R, Brambilla G, Rampoldi A, Rezakovic I, Zavaglia C, Alberti A, Idèo G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Yamamoto K, Masuzawa M, Kato M, Kurosawa K, Kaneko A, Ishida H, Imamura E, Park NJ, Shirai Y, Fujimoto K. Evaluation of combined therapy with chemoembolization and ethanol injection for advanced hepatocellular carcinoma. Semin Oncol. 1997;24:S6-50-S6-S6-50-55. [PubMed] |
| 21. | Bronowicki JP, Vetter D, Dumas F, Boudjema K, Bader R, Weiss AM, Wenger JJ, Boissel P, Bigard MA, Doffoel M. Transcatheter oily chemoembolization for hepatocellular carcinoma. A 4-year study of 127 French patients. Cancer. 1994;74:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Chang JM, Tzeng WS, Pan HB, Yang CF, Lai KH. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer. 1994;74:2449-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Lencioni R, Paolicchi A, Moretti M, Pinto F, Armillotta N, Di Giulio M, Cicorelli A, Donati F, Cioni D, Bartolozzi C. Combined transcatheter arterial chemoembolization and percutaneous ethanol injection for the treatment of large hepatocellular carcinoma: local therapeutic effect and long-term survival rate. Eur Radiol. 1998;8:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Stefanini GF, Amorati P, Biselli M, Mucci F, Celi A, Arienti V, Roversi R, Rossi C, Re G, Gasbarrini G. Efficacy of transarterial targeted treatments on survival of patients with hepatocellular carcinoma. An Italian experience. Cancer. 1995;75:2427-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 25. | Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 26. | Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Eur J Surg Oncol. 2005;31:331-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543-3548. [PubMed] |
| 28. | Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer. 2001;92:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 29. | Greten TF, Papendorf F, Bleck JS, Kirchhoff T, Wohlberedt T, Kubicka S, Klempnauer J, Galanski M, Manns MP. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for t reatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284-290. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Bartolozzi C, Lencioni R, Caramella D, Vignali C, Cioni R, Mazzeo S, Carrai M, Maltinti G, Capria A, Conte PF. Treatment of large HCC: transcatheter arterial chemoembolization combined with percutaneous ethanol injection versus repeated transcatheter arterial chemoembolization. Radiology. 1995;197:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Tanaka K, Nakamura S, Numata K, Okazaki H, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with percutaneous ethanol injection and transcatheter arterial embolization. Radiology. 1992;185:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Koda M, Okamoto K, Miyoshi Y, Kato S, Murawaki Y, Horie Y, Suou T, Kawasaki H. Combination therapy with transcatheter arterial embolization and percutaneous ethanol injection for advanced hepatocellular carcinoma. Hepatogastroenterology. 1994;41:25-29. [PubMed] |
| 34. | Kawai S, Tani M, Okamura J, Ogawa M, Ohashi Y, Monden M, Hayashi S, Inoue J, Kawarada Y, Kusano M. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma--a comparison between L-TAE with farmorubicin and L-TAE with adriamycin: preliminary results (second cooperative study). Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1994;33 Suppl:S97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK