Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.6053
Revised: April 8, 2005
Accepted: April 11, 2005
Published online: October 14, 2005
AIM: To study the function of a-fetoprotein (AFP) in SMMC-7721 hepatoma cells.
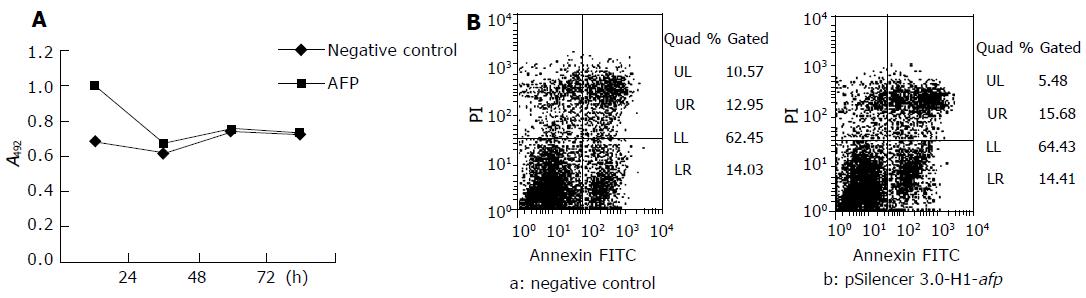
METHODS: A hairpin siRNA expressing plasmid pSilencer3.0-H1-afp was constructed andtransfected into SMMC-7721 cells with Lipofectamine 2000. The expression of AFP was monitored by real-time RT-PCR and immunoassays, its effect on SMMC-7721 cell proliferation and cell death was detected by MTT and fluorescence- activated cell sorter (FACS).
RESULTS: The AFP-siRNA expressing plasmid downregulated the expression of AFP obviously (about 34%), and inhibited SMMC-7721 cell proliferation, but did not induce apoptosis.
CONCLUSION: Downregulation of AFP siRNA inhibits proliferation of SMMC-7721 cells, but cannot cause apoptosis.
- Citation: Wang YS, Ma XL, Qi TG, Liu XD, Meng YS, Guan GJ. Downregulation of alpha-fetoprotein siRNA inhibits proliferation of SMMC-7721 cells. World J Gastroenterol 2005; 11(38): 6053-6055
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/6053.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.6053
Primary hepatocellular carcinoma (PHCC) is one of the most common malignancies in the world, with a high fatality rate and a short survival time[1].
a-fetoprotein (AFP) is a major serum protein produced by the liver or yolk sac in mammals and other vertebrates, and is not detectable in normal adults[2]. However, AFP is often elevated to a significant level in association with development of PHCC, and has been defined as an oncofetal antigen[3,4]. The biological role of AFP has been widely investigated. However, the relationship between AFP and PHCC is still unclear. Some recent investigations indicate that human AFP can enhance the proliferation of mouse hepatoma H-22 and human hepatoma SMMC-7721, BEL-7404, or QGY-7703 cells in vitro[5]. Similar growth stimulatory effect of AFP at low concentrations has also been found in human hepatoma Hep G2 cells. These results have an important implication that AFP may function as a hepatoma growth stimulator, thus suppression of AFP gene expression and its biological activities may become a new strategy for the treatment of AFP-associated tumors such as PHCC.
RNA-mediated interference is the inhibition of expression of specific genes by double-stranded RNAs (dsRNAs)[6]. Since short dsRNAs can silence the expression of its homologous gene in mammalian cells, and do not induce nonspecific interferon response, it is widely used in genome and therapy study. Here we have designed a plasmid pSilencer3.0-H1-afp, which can express siRNAs in mammalian cells to study the function of AFP and its therapeutic effect on hepatoma cells in vitro.
The plasmid pSilencer3.0-H1-afp was constructed as previously described[7]. The targeted sequence of AFP gene (GenBank accession no. NM_005030) is 5-AACTCAGTG-AGGACAAACTAT-3. Positive and negative technical controls were supplied by Ambion Incorporation, and the positive control plasmid was aimed at GAPDH gene.
Human hepatoma SMMC-7721 cells were cultured in RPMI-1640 (Invitrogen) supplemented with 10% FCS. Transfection was done with Lipofectamine 2000 (Invitrogen). One day before transfection, SMMC-7721 cells were seeded in the wells of 24-well culture plates, about 7×104 cells/well. Before transfection, 1 mg plasmid and 2 mL Lipofectamine 2000 were diluted with 50 mL serum-free Opti-MEM I (Invitrogen) and incubated for 5 min. They were mixed and incubated for 20 min at room temperature, added to the medium, mixed gently, and then incubated at 37°C for 4 h. The medium was replaced with a new medium and incubated for 12, 24, and 48 h.
For validation of the silencing effect by quantitative PCR, cDNA was prepared from the SMMC-7721 cell line. RT-PCR was carried out using RT and SYBR Green PCR Master Mix (ABI) according to the manufacturer's instructions. RT reaction was performed at 48°C for 30 min. The cDNA was analyzed by real-time quantitative PCR immediately and stored at -20°Cuntil use. Each PCR was carried out in triplicate in a 25 mL volume for 15 min at 95°C for initial denaturing, followed by 40 cycles at 95°C for 30 s and at 60°Cfor 1 min in the ABI 7000 sequence detection system. Each primer set was first tested to determine optimal concentrations, and products were run out on a 3% agarose gel to confirm the appropriate size. Subsequently, the ABI dissociation curves software was used following a brief thermal protocol (95°C for 15 s and 60°C for 20 s, followed by a slow ramp to 95°C) to control their multiple species in each PCR amplification. cDNA from hepatoma cells was used to construct a standard curve for each gene. Values for each gene were normalized to expression levels of actin-b, and then a ratio comparing the expression in negative and positive control, and pSilencer3.0-H1-afp-transfected hepatoma cells was calculated. The sequences of the primers used for RT-PCR were as follows: actin-b forward: 5-CGT ACC ACT GGC ATC GTG AT-3; reverse: 5-GTG TTG GCG TAC AGG TCT TTG-3; GAPDH forward: 5-CTT CAC CAC CAT GGA GAA GGC-3; reverse primer: 5-GGC ATG GAC TGT GGT CAT GAG-3; AFP forward: 5-AAA TAC ATC CAG GAG AGC CA-3; reverse: 5-CTG AGC TTG GCA CAG ATC CT-3.
AFP concentration in the supernatant in each well was determined by chemiluminescence immunoassay according to the manufacturer抯 instructions.
Eight hours after plasmid transfection, the cells were harvested and replated in 96-well microtiter plates in triplicate at 1×104 cells in 0.1 mL medium/well. Twenty microliters of MTT dye (5 mg/mL) was added and incubated for 0, 24, 48, and 72 h respectively. Following 4 h of incubation at 37°C, formazan crystals were dissolved in dimethyl sulfoxide
(0.1 mL/well). The plates were mechanically agitated for 5 min and the optical density at 540 nm was determined on the microtiter plate reader. Each experiment was done thrice.
Forty-eight hours after transfection, cells were harvested by trypsin digestion, washed twice with cold PBS, and resuspended in 1 binding buffer, regulated to the concentration 1106/mL. Then 100 mL was extracted to a new tube, 5 mL PI and 5 mL annexin V-FITC were added, mixed gently, incubated at 25°C for 15 min. Four hundred microliters of binding buffer was added and analyzed within 1 h with FACS.
To specifically deplete AFP in hepatoma cells, we took advantage of the recently developed vector-based siRNA technology. The targeting sequence of human AFP (GenBank accession no. NM_005030) is 5-AACTCAGTG-AGGACAAACTAT-3. The vector pSilencer3.0-H1-afp, pSilencer3.0-H1-gapdh (positive control) and negative control vector were transfected into SMMC-7721 cells, which were cultured for 48 h, then RT-PCR and AFP in supernatant fluid were assayed. Positive control vector pSilencer3.0-H1-gapdh could silence the GAPDH efficiently. The inhibition rate of the positive control plasmid and pSilencer3.0-H1-afp was 60% and 34% respectively. In order to confirm the inhibitory effect, we assayed the AFP concentration in the supernatant. The AFP level in the supernatant reduced about 40%, 48 h after transfection. These results showed that the expression of AFP in hepatoma cells was downregulated.
Figure 1A shows that vector pSilencer3.0-H1-afp inhibited the proliferation of SMMC-7721 cells obviously compared to the negative control plasmid, and that the suppression effect was most obvious at 32 h. The results of FACS showed no difference between the negative control and pSilencer3.0-H1-afp-treated cells (Figure 1B).
Since AFP was found in the mid-1950s, this oncofetal protein has been used as a PHCC marker in clinical diagnosis[10]. But its biological activities in mammals still remain unclear. To date, only two functional roles of AFP have been ascertained[10]. In the last decade, the growth regulatory properties of AFP have aroused interests as a result of studies involving ontogenetic and oncogenic growth in both cell cultures and animal models. Particularly, the effect of AFP on the development of experimental tumors in mammals has been investigated in vivo with the use of carcinogens[11]. When AFP-treated mice develop larger tumors, they require a longer period for regression and have a significantly higher mortality[10]. Chicken AFP-treated quails develop tumors with shorter latent periods than the untreated quails[8]. The in vivo tumor growth stimulation by AFP can be explained by its immunosuppression. Cell-mediated immunity is an important mechanism of host resistance to malignant neoplasms. Recently, anonymous studies found that antihepatoma effects of AFP antisense S-ODNs are more significant in normal mice than in nude mice[10], suggesting that there exists a relationship between AFP and the susceptibility of hepatoma cells to immunity-mediated cytotoxicity. Recent studies indicate that AFP directly stimulates the proliferation of hepatoma cells in vitro, independent of its immunosuppression[11].
However, the precise relationship between AFP and PHCC is not clear. AFP is highly expressed in hepatic oval cells during the early stages of carcinogenesis, and high levels of AFP in the fully developed PHCC, or in the serum of the host, are associated with more aggressive behaviors, and increased anaplasia[9]. The present study also demonstrated that silencing the expression of AFP could effectively inhibit the growth of hepatoma cells in vitro, suggesting that AFP may play a role in the pathogenesis of PHCC.
The present results indicate that AFP siRNAs expressing plasmid exhibit significant antihepatoma activities in vitro by the sequence-specific silencing of AFP gene expression. But the results of FACS have no obvious difference between the negative control and the pSilencer3.0-H1-afp treated wells. Therefore, the mechanisms of antihepatoma action of AFP siRNAs expressing plasmid are not through the induction of hepatoma cell apoptosis.
The authors express appreciation to Professor Bing-Yu Mao, Kunming Institute of Zoology,Chinese Academy of Sciences and Senior Lecturer Bin Yu, Sydney University, Australia for their critical revision of the manuscript.
| 1. | Colleoni M, Gaion F, Liessi G, Mastropasqua G, Nelli P, Manente P. Medical treatment of hepatocellular carcinoma: any progress? Tumori. 1994;80:315-326. [PubMed] |
| 2. | Wang W, Alpert E. Downregulation of phorbol 12-myristate 13-acetate-induced tumor necrosis factor-alpha and interleukin-1 beta production and gene expression in human monocytic cells by human alpha-fetoprotein. Hepatology. 1995;22:921-928. [PubMed] |
| 3. | Ogata A, Yamashita T, Koyama Y, Sakai M, Nishi S. Suppression of experimental antigen-induced arthritis in transgenic mice producing human alpha-fetoprotein. Biochem Biophys Res Commun. 1995;213:362-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Jacobson HI, Bennett JA, Mizejewski GJ. Inhibition of estrogen-dependent breast cancer growth by a reaction product of alpha-fetoprotein and estradiol. Cancer Res. 1990;50:415-420. [PubMed] |
| 5. | Wang XW, Xie H. Effect of alpha-fetoprotein on the growth of human hepatoma cells in vitro. ShiYan ShengWu XueBao. 1999;32:15-22. [PubMed] |
| 6. | Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10522] [Cited by in RCA: 10327] [Article Influence: 368.8] [Reference Citation Analysis (5)] |
| 7. | Qi TG, Wang YS. Construction and identification of a plasmid expressing SiRNAs in mammalian cells aimed at AFP gene. Shandong Daxue Xuebao. 2004;42:107-109. |
| 8. | Yamada A, Hayami M. Suppression of natural killer cell activity by chicken alpha-fetoprotein in Japanese quails. J Natl Cancer Inst. 1983;70:735-738. [PubMed] |
| 9. | Matsumoto Y, Suzuki T, Asada I, Ozawa K, Tobe T, Honjo I. Clinical classification of hepatoma in Japan according to serial changes in serum alpha-fetoprotein levels. Cancer. 1982;49:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Wang XW, Yuan JH, Zhang RG, Guo LX, Xie Y, Xie H. Antihepatoma effect of alpha-fetoprotein antisense phosphorothioate oligodeoxyribonucleotides in vitro and in mice. World J Gastroenterol. 2001;7:345-351. [PubMed] |
| 11. | Li MS, Li PF, Chen Q, Du GG, Li G. Alpha-fetoprotein stimulated the expression of some oncogenes in human hepatocellular carcinoma Bel 7402 cells. World J Gastroenterol. 2004;10:819-824. [PubMed] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK