Published online Oct 14, 2005. doi: 10.3748/wjg.v11.i38.5920
Revised: May 8, 2005
Accepted: May 12, 2005
Published online: October 14, 2005
AIM: To determine the prognostic significance of isolated tumor cells (ITCs) and lymph node micrometastases in gastric cancer.
METHODS: Hematoxylin and eosin-stained slides of lymph node dissections of 632 consecutive gastric cancers were reviewed. Cytokeratin immunostaining was performed in 280 node-negative cases and 5 cases indefinite for lymph node metastases. Lymph node metastases were divided into ITCs, micrometastases, or macrometastases, according to the sizes of tumor deposits in largest dimension. ITCs were further classified into four groups according to metastasis pattern.
RESULTS: Lymph node metastases were identified by immunostaining in 58 of 280 node-negative cases (20.7%) and were not significantly associated with patient survival (P = 0.3460). After cytokeratin immunostaining, 196 cases were classified as pN1, which consisted of 20 cases with micrometastases detected by immunostaining (pN1mi(i+)), 34 cases with only micrometastases (pN1mi), and 142 cases with pN1 with one or more macrometastases (pN1). Cases with pN1mi and pN1mi(i+) had a significantly better prognosis than the cases with pN1 (P = 0.0037). ITCs were found in 38 of these 58 cases, and could be divided into four groups: 12 cases with only a single cell pattern, 7 cases with multiple individual cells, 5 cases with single small cluster, and 14 cases with multiple small clusters. Among these four groups, cases with ITCs of multiple individual cell pattern showed the worst survival (median survival: 28 mo, P<0.0001).
CONCLUSION: Both size and pattern of lymph node metastases can give prognostic information on the survival of gastric cancer patients.
- Citation: Lee HS, Kim MA, Yang HK, Lee BL, Kim WH. Prognostic implication of isolated tumor cells and micrometastases in regional lymph nodes of gastric cancer. World J Gastroenterol 2005; 11(38): 5920-5925
- URL: https://www.wjgnet.com/1007-9327/full/v11/i38/5920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i38.5920
The most important prognostic factor in patients with gastric cancer is the presence of a regional lymph node metastasis[1]. Some patients with histologically node-negative gastric cancer will die as a result of local or distant tumor recurrence, even though their primary tumor is curatively resected. Adjuvant chemotherapy benefits some patients with node-negative gastric cancer, but the treatment of all node-negative patients is unnecessary, as most node-negative patients will never have recurrence after surgery. Therefore, additional markers would be helpful for predicting patients at risk of a poor prognosis.
For the above purpose, histological evaluations of multiple serial sections of lymph nodes at many levels have been undertaken by several investigators[2,3]. Although serial sectioning can increase the detection of occult lymph node metastases, it is also labor-intensive and expensive for routine diagnostic practice. Furthermore, a single cell or minute cluster cannot be detected even by an expert pathologist. Recent advances in immunohistochemistry and molecular biology allow the identification of discrete and occult tumor cells, which are undetectable by standard hematoxylin and eosin (HE) staining, in the lymph nodes of patients with breast[4,5] esophageal[6,7] and colorectal cancers[8,9]. Many investigators have correlated patient prognosis with the presence of metastases detected by immunostaining, but controversy remains over the importance of single cell or cluster of cancer cells in regional lymph nodes.
Recently, regional lymph node metastasis was further classified into isolated tumor cells (ITCs), micrometastases, or macrometastases according to the size of tumor deposits in largest dimension in patients with breast cancers[10]. ITCs are defined as single cells or small clusters not greater than 0.2 mm in largest dimension, usually with no histologic evidence of malignant activity. Cases with only ITCs are designated as pN0(i+) and categorized into pN0 by AJCC[10]. Single cells and small ill-defined clusters in lymph node metastasis, classified as ITCs, are not uncommon in gastric cancer, since cancer cells in diffuse type cancer frequently loose intercellular adhesion molecules, which represents a critical event of the metastatic cascade[11-13]. However, the clinical importance of size or pattern of metastatic tumor deposits in lymph node metastases is not defined in gastric cancer patients. To clarify the prognostic impact of ITCs and micrometastases on regional lymph nodes, we investigated 632 consecutive gastric cancers resected during one year in the aspect of number, size, and patterns of lymph node metastases and examined their clinical significance.
The files of 659 surgically resected primary gastric cancer cases examined at the Department of Pathology, Seoul National University College of Medicine over a period of 1 year (January 1, 1995-December 31, 1995) were examined in order to evaluate their lymph node metastasis status. Of these 659 cases, 632 cases (95.9%) were available for slide review and immunohistochemical staining of lymph nodes. The series consisted of 280 cases of pN0 stage, 183 cases of pN1 stage, 113 cases of pN2 stage and 56 cases of pN3 stage. Mean age of the patients was 54.5 years, and 92.9% of the patients had undergone curative resection (R0 according to the AJCC guideline). The study included 409 advanced gastric carcinomas and 223 early gastric carcinomas. No patient had received preoperative chemo- or radiotherapy. Glass slides were reviewed to determine histologic types according to WHO classification[11]. The clinical outcomes of the patients were followed from the date of surgery to either the date of death or December 1, 2000, resulting in a follow-up period ranging from 1 to 72 mo (mean: 55 mo). Those cases who lost their follow-up and those who ended in death due to a cause other than gastric cancer were regarded as censored data during survival rate analysis.
HE-stained slides of lymph node dissection of 632 consecutive gastric cancers (31 421 lymph nodes) were reviewed. In contrast to original report, metastatic nodules in the fat adjacent to a gastric carcinoma without evidence of residual lymph node tissue were regarded as regional lymph node metastases by the AJCC guideline. Lymph node metastases were subdivided into three groups according to the AJCC guidelines using the maximum size of tumor deposit, i.e., ITCs are not greater than 0.2 mm in diameter, micrometastases are greater than 0.2 mm but not greater than 2 mm in diameter, and macrometastases are greater than 2 mm in diameter[10]. Lymph nodes with ITCs were not regarded as lymph node metastases, and cases with only micrometastases (not greater than 2 mm in diameter) were classified as pN1mi.
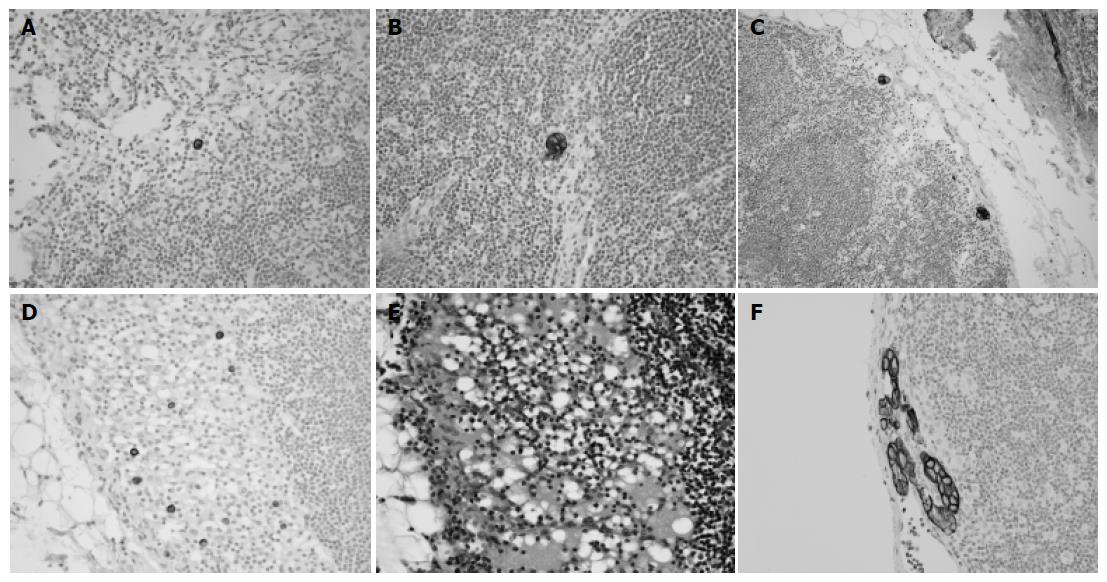
Immunohistochemical staining was performed on 280 gastric cancer cases (9 604 lymph nodes) without regional lymph node metastasis (pN0) by conventional HE staining, and on an additional 5 cases, who were originally reported as pN1 but were indefinite for metastasis by reviewing HE-stained slides. A single serial section (4 mm) was cut from each paraffin block, deparaffinized and dehydrated. Immunostaining with antibodies against cytokeratin (mAb anti-human cytokeratin clone MNF116, DAKO, Glostrup, Denmark) at 1:100 dilution was performed using a streptavidin peroxidase procedure after an antigen retrieval process using microwaves. Cells were considered to be occult lymph node metastases, if they were immunoreactive mainly in their cytoplasmic membranes, were found within the substance of the lymph nodes and were morphologically consistent with cancer cells. Immunohistochemically detected lymph node metastases were also subdivided into three groups: ITCs, micrometastases, or macrometastases, according to the size of tumor deposits in the largest dimension. Tumor deposits in lymph nodes with histologic evidence of malignant activity, such as gland formation or stromal reaction, were defined as micrometastases even if they were smaller than 0.2 mm in maximum dimension. ITCs were subdivided into four groups according to metastasis pattern (single cell, multiple individual cells, single cluster and multiple clusters, Figure 1). The number of lymph nodes containing tumors and the total number of tumor cells or clusters in a lymph node were recorded.
Either the c2 test or Fisher’s exact test (two-sided) was performed to determine the correlation between lymph node metastasis and clinicopathologic parameters. Survival curves were estimated using the Kaplan-Meier product-limit method, and the significance of differences between survival curves was determined using the log-rank test. Results were considered to be statistically significant when P values were less than 0.05. All statistical analyses were conducted using the SPSS 11.0 statistical software program (SPSS, Chicago, IL, USA).
Occult lymph node metastases were identified by cytokeratin immunostaining in 58 (20.7%) of 280 cases or in 157 of 9 604 lymph nodes (1.63%), which were originally reported as pN0. Of the 58 cases with positive occult lymph node metastasis, macrometastasis was found in one case, micrometastases in 19 cases (pN1mi(i+)), and ITCs in 38 cases (pN0(i+)). Four cases, who were originally reported as pN1, were reclassified as pN0, and one case was reclassified as pN1mi(i+) after cytokeratin immunostaining.
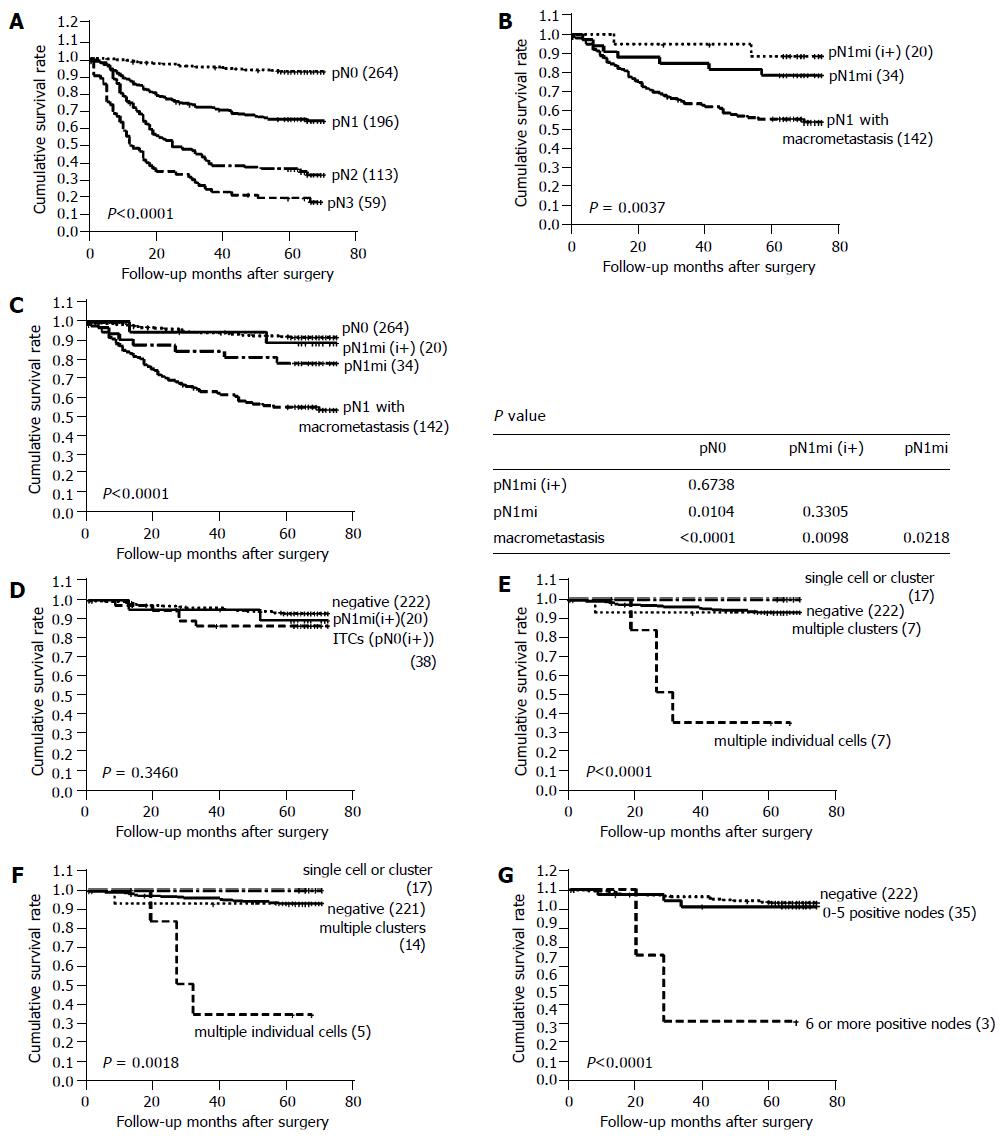
The pN stage, after immunostaining for cytokeratin and reviewing HE-stained slides, was reclassified as pN0 in 264 cases, pN1 in 196 cases, pN2 in 113 cases, and pN3 in 59 cases. The reclassified pN stage was found to be significantly associated with patient survival (Figure 2A).
One hundred and ninety-six cases with pN1 were subdivided into three groups: 20 cases with pN1mi(i+), 34 cases with pN1mi, and 142 cases with pN1 with one or more macrometastases. Cases with pN1mi, including pN1mi(i+), showed a significantly better prognosis than the 142 cases with macrometastases (P = 0.0037, Figure 2B). Furthermore, pN1mi detected by HE staining only was significantly associated with better survival than macrometastases (P = 0.0218, Figure 2C). No significant difference was observed between the survival of cases with pN1mi(i+) and pN1mi (P = 0.3305, Figure 2B). When compared to pN0(i-) cases, pN1mi cases showed significantly poorer survival (P = 0.0104, Figure 2C), but pN1mi (i+) and pN0(i-) cases did not show a survival difference (P = 0.6738).
Cases with lymph node metastases detected by immunoh-istochemistry (pN0(i+) and pN1(i+)) did not show a different survival when compared to cases without lymph node metastases (P = 0.3460, Figure 2D). Patients with immunoh-istochemically detected metastases had poorly differentiated adenocarcinomas more frequently than those without metastases (P = 0.019, Table 1).
| Negative | ITCs | Micro meta-stases | Macro meta-stases | P | ||||
| Single cell | Multiple cells | Single cluster | Multiple clusters | |||||
| WHO (%) | 0.019 | |||||||
| 1W/D, M/D | 112 (50.5) | 6 (50.0) | 0 (0) | 1 (20) | 6 (42.9) | 8 (42.1) | 0 (0) | |
| 2P/D | 54 (24.3) | 4 (33.3) | 7 (100) | 2 (40) | 5 (35.7) | 8 (42.1) | 1 (100) | |
| Mucinous | 9 (4.0) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 2 (10.5) | 0 (0) | |
| 3SRC | 47 (21.2) | 2 (16.7) | 0 (0) | 1 (20) | 3 (21.4) | 1 (5.3) | 0 (0) | |
| Depth (%) | <0.001 | |||||||
| Mucosa | 97 (43.7) | 4 (33.3) | 0 (0) | 2 (40) | 1 (7.1) | 3 (15.8) | 0 (0) | |
| 4SM | 72 (32.4) | 2 (16.7) | 1 (14.4) | 1 (20) | 4 (28.7) | 5 (26.3) | 1 (100) | |
| 5PM | 21 (9.5) | 1 (8.3) | 0 (0) | 0 (0) | 1 (7.1) | 2 (10.5) | 0 (0) | |
| Subserosa | 28 (12.6) | 5 (41.7) | 3 (42.8) | 2 (40) | 7 (50) | 4 (21.1) | 0 (0) | |
| Serosa | 4 (1.8) | 0 (0) | 3 (42.8) | 0 (0) | 1 (7.1) | 5 (26.3) | 0 (0) | |
| Total (%) | 222 (100) | 12 (100) | 7 (100) | 5 (100) | 14 (100) | 19 (100) | 1 (100) | |
Four groups of ITCs included 12 cases with a single cell pattern, 7 cases with multiple individual cells, 5 cases with a single cluster and 14 cases with multiple clusters. Among them, cases with multiple individual cells showed the poorest survival (median survival: 28 mo, P<0.0001, Figure 2E), and had poorly differentiated non-solid type adenocarcinomas with infiltrative border. Lymphatic invasion was not found in these cases. Histologically, cytokeratin-positive individual cells were found in the sinus of lymph nodes, and these could not be recognized by routine HE staining (Figure 1). In four of seven cases with multiple individual cells, the maximum number of tumor cells in a single lymph node exceeded 20 (Table 2), and prognosis was grave especially in these four cases. By Cox regression analysis, the pattern of metastasis was found to be a significant prognostic factor independent of pT stage and histologic type (P = 0.018, Table 3). Two of the seven patients with multiple individual cells had stage IV disease, and the pattern of multiple individual cells was associated with poor survival even after the patients with stage IV disease were deleted (P = 0.0018, Figure 2F).
| Prognostic factor | Hazard ratio (95%CI) | P |
| Pattern of ITCs | 0.018 | |
| Multiple individual cells vsothers | 4.09 (1.28-13.10) | |
| PT stage | <0.001 | |
| II-IV vs I | 8.77 (2.89-26.32) | |
| Lauren's classification | 0.276 | |
| Diffuse vs intestinal | 1.73 (0.65-4.61) |
Thirty-five of thirty-eight cases with ITCs had one to five positive metastatic nodes. The remaining cases had six or more positive metastatic nodes (9, 11, and 19 metastatic lymph nodes, respectively). Cases with ITCs in six or more lymph nodes showed a significantly poorer prognosis than those with ITCs in 1-5 lymph nodes (P<0.0001, Figure 2G).
When examining gastric cancer and related material, the pathologist aims to achieve as simply and reliably as possible, a comprehensive diagnosis and prognosis of therapeutic relevance. The routine histologic examination of lymph nodes has long been the gold standard for identifying metastases to regional lymph nodes resected as part of the staging procedure for tumors in diverse locations, including the stomach. However, this method has an inherent limitation, the potential for sampling error. Serial sectioning has been employed to scrutinize metastases[14], but it is impractical for routine surgical pathology practice. In this study, we performed immunohistochemical staining against cytokeratin on routine sections in the cases with original diagnosis of pN0. We found that micrometastases or macrometastases were overlooked in 20 of 280 node-negative cases (7.14%), and ITCs were found in 38 cases (13.6%).
The prognostic impact of lymph node metastases detected by immunohistochemistry remains controversial in gastric cancer. Some authors have reported an insignificant correlation between lymph node metastasis detected by immunostaining and patient survival[15-17], but others have reported a significant impact on patient survival[18-21]. In contrast to previous reports, we subdivided lymph node metastases according to the size of tumor deposits, and then subdivided cases with ITCs into four groups according to the pattern of tumor deposits. In this large scale study, lymph node metastases as detected by immunostaining were not significantly associated with patient survival.
Ishida et al[21] have classified lymph node metastases into five groups according to the pattern of metastasis (single cell vs cluster), but did not analyze the implications on patient survival. Fukagawa et al[17] demonstrated that the pattern of micrometastases (single cell vs cluster) does not affect the survival curve. In the present study, ITCs were classified into four groups: single cell, multiple individual cells, single cluster, and multiple clusters. Among ITCs, only cases with multiple individual cells comprised the poor prognosis group. Tumors with a pattern of multiple individual cells showed non-solid poorly differentiated type adenocarcinoma and had infiltrative border. Although the pattern of multiple individual cells was very uncommon (7 of 280 pN0, 2.5%), this pattern had a significant impact on patient survival and showed a distinct histologic feature. Therefore, cytokeratin immunostaining is recommended in node-negative gastric cancers especially in non-solid poorly differentiated type adenocarcinoma with infiltrative border, to identify occult metastasis with multiple individual cell pattern.
According to the cancer staging of AJCC, micrometastases are defined when the size of tumor deposits in largest dimension is greater than 0.2 mm and not greater than 2 mm in diameter, and cases in which only micrometastases are detected are defined as pN1mi in breast cancer. However, in previous reports on gastric cancer[17-21], micrometastases are defined as lymph node metastases detected by immunohistochemistry, not according to the size of tumor deposits. In the present study, micrometastases were defined according to cancer staging method recommended by AJCC. Cases with pN1mi including pN1mi(i+) showed a significantly better prognosis than cases with pN1 with macrometastases (P = 0.0037), and pN1mi by only HE staining was also significantly associated with better survival than macrometastases (P = 0.0218). Therefore, we recommend that pN1mi should be defined in gastric cancer as is currently done in breast cancer. Furthermore, the survival of patients with pN1mi(i+) was not significantly different from that of patients with pN0. Therefore, we also recommend classifying pN1mi(i+) separately from pN1mi.
Several lines of evidence support the interpretation that keratin-positive cells in lymph nodes represent metastatic carcinoma, though occasional normal constituents of lymph nodes may be stained positively for cytokeratin. Other than cancer cells, undesirable cytokeratin has been reported both in plasma cells[22,23] and in interstitial reticulum cells[22,24]. Cytokeratin-positive metastatic cells have an epithelial morphology, in contrast to the dendritic process appearance of keratin-positive reticulum cells. Plasma cells show weak and cytoplasmic expression of cytokeratin and have their characteristic features. Cells are considered as occult node metastases only when they show strong and membranous expression of cytokeratin, reside within the substance of the lymph nodes, and exhibit epithelial morphology.
In conclusion, immunohistochemical staining against cytokeratin can detect occult metastases in regional lymph nodes of patients with gastric cancer, and the presence of immunohistochemically detected ITCs does not affect patient survival, except for a subset of cases with metastasis of multiple individual cells. pN1mi or pN1mi(i+) is distinct from pN0 or pN1 in the aspect of patient survival. Therefore, the size and pattern of lymph node metastases can provide prognostic information in gastric cancer.
| 1. | Adachi Y, Kamakura T, Mori M, Baba H, Maehara Y, Sugimachi K. Prognostic significance of the number of positive lymph nodes in gastric carcinoma. Br J Surg. 1994;81:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Isozaki H, Okajima K, Fujii K. Histological evaluation of lymph node metastasis on serial sectioning in gastric cancer with radical lymphadenectomy. Hepatogastroenterology. 1997;44:1133-1136. [PubMed] |
| 3. | Prognostic importance of occult axillary lymph node micrometastases from breast cancers. International (Ludwig) Breast Cancer Study Group. Lancet. 1990;335:1565-1568. [PubMed] |
| 4. | Cote RJ, Peterson HF, Chaiwun B, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Gusterson B, Neville AM. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet. 1999;354:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 358] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Dowlatshahi K, Fan M, Snider HC, Habib FA. Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer. 1997;80:1188-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Glickman JN, Torres C, Wang HH, Turner JR, Shahsafaei A, Richards WG, Sugarbaker DJ, Odze RD. The prognostic significance of lymph node micrometastasis in patients with esophageal carcinoma. Cancer. 1999;85:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Doki Y, Ishikawa O, Mano M, Hiratsuka M, Sasaki Y, Kameyama M, Ohigashi H, Murata K, Yamada T, Miyashiro I. Cytokeratin deposits in lymph nodes show distinct clinical significance from lymph node micrometastasis in human esophageal cancers. J Surg Res. 2002;107:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 409] [Article Influence: 14.6] [Reference Citation Analysis (16)] |
| 9. | Nicholson AG, Marks CG, Cook MG. Effect on lymph node status of triple levelling and immunohistochemistry with CAM 5.2 on node negative colorectal carcinomas. Gut. 1994;35:1447-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | American Joint Committee on Cancer. AJCC cancer staging manual 6th ed. New York: Springer-Verlag 2002; . |
| 11. | International Agency for Research on Cancer (IARC). World Health Organization Classification of Tumors; pathology and genetics of tumors of the digestive system. Lyon: IARC Press 2000; . |
| 12. | Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH. Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer. 2001;95:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Kumar V, Abbas AK, Fausto N. Robbins and Cotran pathologic basis of disease 7th ed. Philadelphia: Elsevier 2004; . |
| 14. | Pickren JW. Significance of occult metastases. A study of breast cancer. Cancer. 1961;14:1266-1271. [PubMed] |
| 15. | Morgagni P, Saragoni L, Scarpi E, Zattini PS, Zaccaroni A, Morgagni D, Bazzocchi F. Lymph node micrometastases in early gastric cancer and their impact on prognosis. World J Surg. 2003;27:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 16. | Choi HJ, Kim YK, Kim YH, Kim SS, Hong SH. Occurrence and prognostic implications of micrometastases in lymph nodes from patients with submucosal gastric carcinoma. Ann Surg Oncol. 2002;9:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Fukagawa T, Sasako M, Mann GB, Sano T, Katai H, Maruyama K, Nakanishi Y, Shimoda T. Immunohistochemically detected micrometastases of the lymph nodes in patients with gastric carcinoma. Cancer. 2001;92:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer. 2002;94:2867-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Nakajo A, Natsugoe S, Ishigami S, Matsumoto M, Nakashima S, Hokita S, Baba M, Takao S, Aikou T. Detection and prediction of micrometastasis in the lymph nodes of patients with pN0 gastric cancer. Ann Surg Oncol. 2001;8:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Ishida K, Katsuyama T, Sugiyama A, Kawasaki S. Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer. 1997;79:1069-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Xu X, Roberts SA, Pasha TL, Zhang PJ. Undesirable cytokeratin immunoreactivity of native nonepithelial cells in sentinel lymph nodes from patients with breast carcinoma. Arch Pathol Lab Med. 2000;124:1310-1313. [PubMed] |
| 23. | Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Gould VE, Bloom KJ, Franke WW, Warren WH, Moll R. Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch. 1995;425:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK