Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5688
Revised: April 1, 2005
Accepted: April 2, 2005
Published online: September 28, 2005
AIM: As intraductal papillary mucinous neoplasm (IPMN) has a favorable prognosis, associated malignancies have potential significance in these patients. We examined the incidence and characteristics of pre-existing, coexisting and subsequent malignancies in patients with IPMN.
METHODS: Seventy-nine cases of IPMN were diagnosed by detection of mucous in the pancreatic duct during endoscopic retrograde pancreatography. Histological diagnosis was confirmed in 30 cases (adenoma (n = 19) and adenocarcinoma (n = 11). Other primary malignancies associated with IPMN, occurring in the prediagnostic or postdiagnostic period, were investigated. Postdiagnostic follow-up period was 3.3 ± 0.5 years (range, 0.2-20 years).
RESULTS: Other 40 malignancies occurred in 28 patients (35%). They were found before (n = 15), at (n = 19) and after (n = 6) the diagnosis of IPMT. Major associated malignancies were gastric cancer (n = 12), colonic cancer (n = 7), esophageal cancer (n = 4), pulmonary cancer (n = 4), and independent pancreatic cancer (n = 3). Pancreatic cancer was synchronous with IPMN in two patients and metachronous in one (3 years after diagnosis of IPMN). Thirty-one lesions were treated surgically or endoscopically. Fourteen patients died of associated cancers. Development of other malignancies was related to age (71.9 ± 8.2 vs 66.8 ± 9.3, P < 0.05), but not to gender or site of the tumor.
CONCLUSION: IPMN is associated with a high incidence of other malignancies, particularly gastric and colonic cancers. Common genetic mechanisms between IPMN and other associated malignancies might be present. Clinicians should pay attention to the possibility of associated malignancies in preoperative screening and follow-up of patients with IPMN.
- Citation: Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol 2005; 11(36): 5688-5690
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5688
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a distinct entity characterized by intraductal papillary growth and thick mucous secretion. Copious mucous fills the main and branch pancreatic ducts and causes ductal dilatation. IPMN histologically shows a broad spectrum ranging from adenoma to invasive carcinoma. IPMN is classified into two subtypes of main duct type and branch duct type based on the site of tumor involvement. IPMN is a slow-growing tumor and many cases of branch duct type IPMN have been followed. IPMN, even when malignant, is often resectable, and has a favorable prognosis, compared with that of pancreatic ductal adenocarcinoma[1-3]. Prolonged postoperative survival can be anticipated in many IPMN cases. There are few papers reporting that IPMN is sometimes accompanied by malignant diseases of the other organs or pancreatic adenocarcinoma[4,5]. This report thus evaluates the incidence and features of associated malignancies in patients with IPMN.
Between 1980 and 2004, 79 patients with IPMN were treated in Tokyo Metropolitan Komagome Hospital. They were diagnosed by detection of mucous in the pancreatic duct during endoscopic retrograde pancreatography. These patients comprised 54 males and 25 females, with an age of 68.5 ± 9.2 (mean±SD) years. Twenty-three patients underwent surgical resection. Thirteen patients became unclear during follow up (1 -5 years). Histological examination of the surgical (n = 23), biopsied (n = 2), and autopsied (n = 5) specimens revealed adenoma in 19 patients, non-invasive adenocarcinoma in 6, and invasive adenocarcinoma in 5. They were classified into main duct type (n = 30) and branch duct type (n = 49) based on the site of tumor involvement. Postdiagnostic follow-up period was 3.3 ± 0.5 years (range, 0.2-20 years).
The inpatient records of these 79 patients were reviewed mainly for previous and coexisting nonpancreatic and independent pancreatic neoplasms found at the time of diagnosis of IPMN. Furthermore, postdiagnostic follow-up data were obtained from outpatient charts.
Statistical analysis was performed using Student’s t-test and Fisher’s exact probability test. Differences were considered significant at P < 0.05.
Other 40 malignancies were diagnosed in 28 (35%) of 79 patients with IPMN. They were found before (n = 15), at (n = 19) and after (n = 6) the diagnosis of IPMN. Major associated malignancies were gastric cancer (n = 12), colonic cancer (n = 7), esophageal cancer (n = 4), pulmonary cancer (n = 4), and independent pancreatic cancer (n = 3) (Table 1). Three other organs were involved in three patients, and two organs in four patients (Table 2). Double gastric cancers occurred in two patients. All of 15 other malignancies occurring 1-10 years (3.7 ± 3.6 years) before the diagnosis of IPMN were resected surgically or endoscopically. Fifteen of nineteen other malignancies diagnosed simultaneously with IPMN were resected surgically or endoscopically. Only one other malignancy diagnosed after IPMN was surgically resected. Fourteen patients died of associated malignancies. Age at IPMN diagnosis in patients with other malignancies was significantly older than that in those without other malignancies (P < 0.05). However, gender, follow-up period, site of IPMN, and resection of IPMN did not differ significantly between the two groups (Table 3).
| Previous | At diagnosis | Post diagnosis | Total | |
| Gastric cancer | 4 | 7 | 1 | 12 |
| Colonic cancer | 4 | 3 | 0 | 7 |
| Esophageal cancer | 3 | 1 | 0 | 4 |
| Pulmonary cancer | 0 | 2 | 2 | 4 |
| Pancreatic cancer | 0 | 2 | 1 | 3 |
| Breast cancer | 2 | 0 | 0 | 2 |
| Hepatocellular cancer | 0 | 2 | 0 | 2 |
| Uterine cancer | 1 | 0 | 0 | 1 |
| Pharyngeal cancer | 1 | 0 | 0 | 1 |
| Bile duct cancer | 0 | 1 | 0 | 1 |
| Prostatic cancer | 0 | 1 | 0 | 1 |
| Laryngeal cancer | 0 | 0 | 1 | 1 |
| Malignant lymphoma | 0 | 0 | 1 | 1 |
| Case | Age (yr) | Sex | Previous | At diagnosis | Postdiagnosis |
| 1 | 76 | M | Gastric cancer (EMR, 1 yr ago) | Pulmonary cancer (4 yr after) | |
| Esophageal cancer (EMR, 1 yr ago) | |||||
| 2 | 74 | F | Uterine cancer (ope, 10 yr ago) | Hepatocellular cancer | |
| Colonic cancer | |||||
| 3 | 70 | M | Gastric cancer | ||
| Colonic cancer | |||||
| Pulmonary cancer | |||||
| 4 | 83 | F | Esophageal cancer | Malignant lymphoma (1 yr after) | |
| 5 | 78 | M | Hepatocellular cancer | Gastric cancer (6 yr after) | |
| 6 | 78 | M | Esophageal cancer (EMR, 1 yr ago) | Laryngeal cancer (6 yr after) | |
| 7 | 68 | M | Esophageal cancer (EMR, 2 yr ago) | Pancreatic cancer (3 yr after) |
| With other malignancies (n = 28) | Without other malignancies (n = 51) | P | |
| Age (yr) | 71.9±8.2 | 66.8±9.3 | P<0.05 |
| Male/Female | 8/20 | 34/17 | NS |
| Follow-up period1 | 3.2±0.5 | 3.4±0.5 | NS |
| Site of IPMN | NS | ||
| Main duct | 13 | 17 | |
| Branch duct | 15 | 34 | |
| Resection/Nonresection of IPMN | 7/20 | 16/35 | NS |
| Cause of death | |||
| Invasive IPMN | 1 | 4 | |
| Gastric cancer | 4 | 0 | |
| Pulmonary cancer | 3 | 0 | |
| Pancreatic cancer | 2 | 0 | |
| Colonic cancer | 2 | 0 | |
| Esophageal cancer | 1 | 0 | |
| Hepatocellular cancer | 1 | 0 | |
| Malignant lymphoma | 1 | 0 | |
| Pneumonia | 1 | 1 | |
| Renal failure | 0 | 1 |
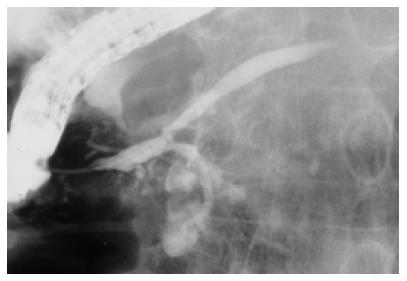
Three patients had both IPMN and independent pancreatic cancer. Two patients had the two lesions synchronously, and pancreatic cancer occurred 3 years after the diagnosis of IPMN in one patient. All the three IPMNs were of branch type located in the pancreatic head and ranged from 1.5 to 3 cm. The lesion of IPMN was proximal to pancreatic cancer in two patients and distal in the other. Independence of IPMN and pancreatic cancer of two patients was proved histologically in the resected and autopsied pancreas, respectively. Two lesions were radiologically far from each other in the other patient (Figure 1). Although prognosis was poor in two patients in whom the presence of pancreatic cancer led to the diagnosis of IPMN, one patient with pancreatic cancer discovered during the follow-up of IPMN is doing well without any signs of recurrence, 36 mo after the operation (Table 4).
| Case | Age (yr) | Sex | Syn/Met | Othermalignancies | IPMNlocation | Site | Therapy | Pancreaticlocation | Cancertherapy | Stage | Prognosis |
| 1 | 71 | M | Syn | None | Head | Branch | None | Body | DP | III | Died 7 mo after operation |
| 2 | 75 | M | Syn | None | Head | Branch | None | Head | None | IV | Died 8 mo after diagnosis |
| 31 | 68 | M | Met | Esophageal cancer | Head | Branch | PD | Head | PD | II | Alive now 3 yr after diagnosis of IPMN |
The incidence of occurrence of multiple primary malignant neoplasms occurring in different tissues has been reported as 5.1%[6]. The incidence of other malignant neoplasms preceding, following or occurring simultaneously with the diagnosis of pancreatic cancer was reported to be 7%[4]. The present study revealed a high incidence of other malignancies (35%) in patients with IPMN. Other 40 malignancies were found before (n = 15), at (n = 19) and after (n = 6) the diagnosis of IPMN. Major associated malignancies were gastric cancer (n = 12) and colonic cancer (n = 7). Sugiyama and Atomi[4] reported that 15 (32%) of 42 patients with IPMN had nonpancreatic malignancies, including colonic cancer (n = 5) and gastric cancer (n = 4). Yamaguchi et al[5] also reported that 13 (27%) of 48 patients with IPMN had malignant diseases, including gastric cancer (n = 5) and colonic cancer (n = 2). Incidence and associated other malignancies were similar to our results. In the present series, 14 patients died of associated malignancies. As IPMN is a slow-growing tumor with a relatively favorable prognosis, associated malignancies have potential prognostic significance. Systemic surveillance at the diagnosis of IPMN and careful follow-up is necessary for early detection of associated malignancies in patients with IPMN.
Interestingly, three patients with IPMN had also independent pancreatic cancer. All these IPMNs were of branch type located in the pancreatic head. The lesion of IPMN was proximal to pancreatic cancer in two patients and distal in the other. Pancreatic cancer occurred during the follow-up of IPMN could be diagnosed in relatively early stage. There is only one report describing the relationship between IPMN and pancreatic cancer[7]. In this report, independent pancreatic cancer was detected in 7 (10%) of 69 patients with IPMN, suggesting that the entire ductal epithelium of the pancreas with IPMN might already be in the premalignant condition.
The cause of frequent development of other malignancies in patients with IPMN is unknown. In the present study, age at IPMN diagnosis in patients with other malignancies was significantly older than the age in those without other malignancies. As elderly patients are more susceptible to neoplasms in most organs than younger patients and IPMN mainly affects elderly men, patients with IPMN have increased risk for other malignancies due to their age. Furthermore, oncogenetic changes of various organs of patients with IPMN might induce frequent development of other malignancies. Recent study has suggested that IPMN might form part of the spectrum of lesions encountered in attenuated familial adenomatous polyposis[8]. Common underlying genetic etiology might be present between IPMN and other associated malignancies. Further investigations focusing on this field are necessary in future.
In conclusion, IPMN is associated with a high incidence of other malignancies including pancreatic cancer. As IPMN has a relatively favorable prognosis, associated malignancies have potential prognostic significance. Clinicians should pay attention to the other associated malignancies in patients with IPMN.
| 1. | Obara T, Maguchi H, Saitoh Y, Itoh A, Arisato S, Ashida T, Nishino N, Ura H, Namiki M. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993;88:564-569. [PubMed] |
| 2. | Kimura W, Sasahira N, Yoshikawa T, Muto T, Makuuchi M. Duct-ectatic type of mucin producing tumor of the pancreas--new concept of pancreatic neoplasia. Hepatogastroenterology. 1996;43:692-709. [PubMed] |
| 3. | Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997;122:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Yamaguchi K, Yokohata K, Noshiro H, Chijiiwa K, Tanaka M. Mucinous cystic neoplasm of the pancreas or intraductal papillary-mucinous tumour of the pancreas. Eur J Surg. 2000;166:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Moertel CG, Dockerty MB, Baggenstoss AH. Multiple primary malignant neoplasms. I. Introduction and presentation of data. Cancer. 1961;14:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Chetty R, Salahshor S, Bapat B, Berk T, Croitoru M, Gallinger S. Intraductal papillary mucinous neoplasm of the pancreas in a patient with attenuated familial adenomatous polyposis. J Clin Pathol. 2005;58:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK