Published online Sep 28, 2005. doi: 10.3748/wjg.v11.i36.5655
Revised: June 2, 2005
Accepted: June 6, 2005
Published online: September 28, 2005
AIM: To observe the status of tumor-associated B7 molecule mRNA expression in human colorectal cancer tissue by in situ hybridization.
METHODS: The mRNA expression patterns of cancer-associated B7-1,B7H1,B7H2,ICOS in 22 specimens of human colorectal cancer tissue were monitored by in situ hybridization (ISH) with digoxin-labeled oligonucleotide probes.
RESULTS: B7-1,B7H1,B7H2,ICOS mRNA were detected in both cancer cells and tumor infiltrating lymphocytes (TIL). The mRNA expression level of these molecules in tumor cells was higher than that in TIL (0.76 ± 0.54-1.62 ± 0.82 vs 0.38 ± 0.19-0.65 ± 0.33, P<0.001). There was no relationship between expression level of tested B7 family molecules and patients’ sex, age, differentiation status of cancer and regional lymph node metastasis.
CONCLUSION: Th2 cytokine predominant in tumor microenvironment might be related to the expression of B7H1,B7H2 co-signal molecules in tumor cells and TIL.
- Citation: Xiao JX, Bai PS, Lai BC, Li L, Zhu J, Wang YL. B7 molecule mRNA expression in colorectal carcinoma. World J Gastroenterol 2005; 11(36): 5655-5658
- URL: https://www.wjgnet.com/1007-9327/full/v11/i36/5655.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i36.5655
Although it has been well accepted that human tumor is immunogenic, most patients suffering from cancer are destined to die due to tumor progress. Establishment of the bisignal model for T-cell activation leads people to think that inactivation of infiltrating immune potential cells in tumor tissue might be immune anergic owing to the deficiency of co-stimulatory molecules. Tumor cells modified with B7 co-stimulatory molecule gene could be rejected by tumor bearing host[1,2], but most human cancer tissues express co-stimulators[3,4], suggesting that co- stimulatory molecules might not be the only mechanism of immune evasion. The tumor-associated B7H1 and B7H2, new members of B7 family can preferentially stimulate the production of IL-10, promote activated T cell apoptosis[5].
In the present study, the status of tumor-associated B7 molecule mRNA expression in human colorectal cancer tissue was observed using B7H1 , B7H2 and ICOS cDNA probe, in situ hybridization. The results showed that B7-1, B7H1 , B7H2, ICOS mRNA were expressed in both cancer cells and tumor infiltrating lymphocytes (TIL), indicating that Th2 cytokine predominant in tumor microenvironment might be related to the expression of B7H1, B7H 2 co-signal molecules in tumor cells and TIL. Elucidation of tumor-associated B7 molecules may contribute to the design of T cell-based cancer immunotherapy.
Tissue samples were obtained from 22 patients with colorectal cancer. The specimen were fixed in 4 g/L formaldehyde in phosphate-buffered saline (PBS) immediately and embedded in paraffin. Serial sections (5 μm in thickness) were cut for in situ hybridization or histological evaluation, and mounted on slides covered with APES, dried overnight at 65°C, stored at -70°C until use. The diagnosis of colorectal carcinoma was histopathologically verified in all cases. None of the patients had previously received radio-, chemo-, or immunotherapy.
Primer 3 software was used to design oligonuleotide probes complementary to the mRNA of all kinds of target sequences including B7-1, B7H1, B 7H2, ICOS mRNA. The specificity of all oligonucleotide probes was analyzed by BLAST software (http://www.ncbi.nih.gov). The probes were labeled by tailing the oligonucleotides with digoxigenin-11-dUTP kit (Boehringer Mannheim, German). A labeling activity of 1.56 nmol/L was obtained (Primer3 http://www. genome.wi.mit.edu/cgi-bin/primer/Primer3;BLAST http://www.ncbi.nlm.gov/cgi-bin/BLAST). Sequence of oligonuleotide probes of co-stimulatory molecules was tested (B7-1: 5’-CAT GAA GCT GTG GTT GGT TG -3’; B7H1: 5’-TGC TTG TCC AGA TGA CTT CG -3’; B7H2: 5’-CCA TCG CTC TGA CTT CCT TC -3’; ICOS: 5’-TTC AGC TGG CAA CAA AGT TG-3’).
Diethyl pyrocarbonate (DEPC) water was used for all solutions necessary for ISH. The sections were deparaffinized in xylene and rehydrated in descending ethanol, followed by digestion with 1 g/L proteinase K at 37°C for 30 min and terminated with 20 g/L glycine in PBS for 5 min. Then, the sections were refixed in 40 g/L polyformaldehyde for 20 min, washed with PBS for 10 min, treated with 0.2 mol/L HCI for 10 min and washed with DEPC water for 3 min. The sections were dehydrated in ascending gradient ethanol, air-dried and followed by prehybridization at 42°C for 2 h. Hybridization reaction was carried out at 42°C for 22 h. Then the sections were washed with gradient SSC thoroughly and followed by treatment with digoxin antibody at 37°C for 2 h. The color was developed in NBT-BCIP substrate, then counterstained with 10 g/L methyl green in distilled water, dehydrated and mounted with neutral gum.
For negative controls, probes and antibodies were replaced by PBS or the slides were treated with RNase A (20 mg/L) at 37°C for 30 min. B7-1 mRNA expression in mononuclear leucocytes was used as positive control.
Purple blue precipitation in cytoplasm under light microscope was considered as positive signal, and 5 high power fields were randomly chosen from each slide, The percentage of positive cells and the positive cell index (total positive granule number/positive cell number) were calculated. Intensity of the color reaction was classified into 4 grades: strongly positive (+++), positive (++), weakly positive (+) and negative (-) and scored as 3, 2, 1 and 0, respectively. The Accumulation index was determined as percentage of positive cells multiply intensity score.
All datas were expressed as mean±SD. Analysis was performed by using chi-square test and Spearman correlation analysis with SPSS 11.0 software. P<0.05 was considered statistically significant.
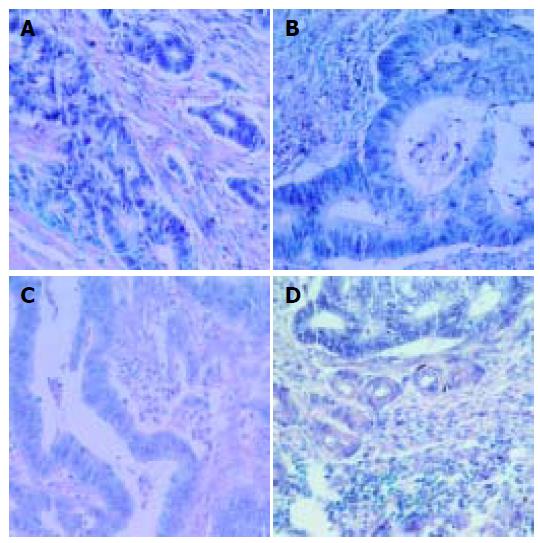
Expression of B7-1, B7H1, B7H2, ICOS mRNA was detected in both cancer cells and tumor infiltrating lymphocytes (Figure 1) . The mRNA expression level of these molecules in tumor cells was higher than that in TIL (0.76 ± 0.54-1.62 ± 0.82 vs 0.38 ± 0.19-0.65 ± 0.33, P = 0.000). However, when the expression of B7 family mRNA was analyzed with respect to the tested members, there was a significant difference in B7H1 and ICOS expression between TIL and cancer cells (P<0.05) . The expression of B7H1 and ICOS mRNA was higher in tumor cells and TIL. The expression of B7 family molecules either in tumor cells or in TIL was not correlated with patients’ sex, age, differentiation status of cancer and regional lymph node metastasis. B7H1 expression in TIL and tumor invasiveness was significantly associated with the intensity and the depth of tumor invasion (P = 0.050, Table 1, Table 2, Table 3).
| B7 molecule | Tumor cell | TIL | ||||
| T2 | T3 | T4 | T2 | T3 | T4 | |
| B7-1: | 1.22 ± 0.82 | 1.28 ± 0.70 | 1.35 ± 0.42 | 0.36 ± 0.43 | 0.50 ± 0.25 | 0.43 ± 0.24 |
| B7H1: | 1.43 ± 0.63 | 1.55 ± 0.76 | 1.01 ± 0.63 | 0.66 ± 0.25 | 0.76 ± 0.36a | 0.42 ± 0.21 |
| B7H2: | 0.62 ± 0.05 | 0.91 ± 0.69 | 0.56 ± 0.28 | 0.42 ± 0.21 | 0.39 ± 0.19 | 0.33 ± 0.18 |
| ICOS: | 1.59 ± 1.14 | 1.77 ± 0.78 | 1.35 ± 0.78 | 0.48 ± 0.35 | 0.53 ± 0.23 | 0.59 ± 0.33 |
It has been accepted that the efficacy of antitumor immunity is mainly affected by three factors, namely status of tumor immunogenecity, expression of co-stimulatory molecules and type of cytokines. Lack of co -stimulatory molecules in tumor cells might be a reasonable explanation of the tumor immune evasion, but the fact that most human cancer tissues express co-stimulators makes it contradictory. Si et al[3] reported that B7 -1 molecule expresses at least in more than half of human solid tumors. MHC-I, II molecules are detectable in human cervical cancer and tumor-associated dendritic cell (DC)[4]. Analysis of tumor microenvironment has demonstrated that type II cytokines are predominant in non-small cell lung cancer and gastric carcinoma[5,6]. Interestingly there is a dynamic change with the progress of tumor, i.e. the expression of IFN-γ and IL-2 decreases, whereas the expression of Th2 cytokines such as IL-4, IL-10 and TNF-β increases significantly.
There is evidence that co-stimulators play a very important role in antigen-specific T cell activation. In vitro and in vivo experiments have shown that T cell interaction via T-cell receptor (TCR) with MHC/antigen complexes in the absence of co-stimulation may lead to induction of anergy rather than activation. B7-1 gene-modified tumor cells can elicit tumor rejection response in xenograft tumor models. All these suggest that deficiency of co-stimulators is the key factor for immune evasion. However, Dong et al[7] found that CD28 by its natural legend B7- 1 or B7-2 triggers Th1 cytokine secretion including IFN-γ and IL-2, while IL-10 production is increased considerably after stimulation with immobilized B7H 1Ig and antibody against CD3. Dynamic observation has demonstrated that the production of IL-2 peaks 24 h after B7H1 co-stimulation, whereas IL-10 secretion starts to increase after 48 and 72 h. Additionally, monoclonal antibody against IL-2 could inhibit IL-10 secretion from T cells co-stimulated with B7H1Ig. ICOS, one of the B7 legends, plays a critical and non-dispensable role in T cell activation and proliferation in vitro, especially in regulating Th2 differentiation. In addition, it is critical for efficient T-cell priming and production of Th2 cytokines in vivo , in particular IL-4 and IL-10. Results obtained from some experiments demonstrated that Ag stimulation of naive CD4+ T cells in the absence of ICOS signal results in impaired Th2 development[8]. At the molecular level, ICOS is involved in upregulating the expression of CD40L as well as promoting the secretion of IL-4 and IL-13. ICOS is a co-stimulatory receptor essential for T- cell activation and function and also plays an important role in acquired immunity[9,10]. This could well elucidate our previous findings, i.e. although expression of B7-1 by human tumor cells can activate T cells and enhance the secretion of Th1 cytokines such as IL-2 and IFN-γ , Th2 cytokine predominates ultimately since co-stimulators such as B7H1, B7H2 and ICOS act dominantly.
In the present study, we observed that B7-1 co-stimulatory molecule mRNA was expressed in tumor cells and tumor infiltrating lymphocytes (TIL) of human colorectal carcinoma by in situ hybridization, which is consistent with many experiments of B7-gene transfection vaccine[11,12]. However, the other members of B7 family such as B7H1, and ICOS can also be detected. In our study, the expression of B7 molecules in tumor cells was higher than that in TIL (P<0.005), and B7H1 and ICOS mRNA expression in tumor cells was even higher than that of B7-1, suggesting that B7 family plays a role in tumor immunity. In addition, the expression of B7H1 and ICOS mRNA is related with the invasion depth of tumor, the mRNA expression of B7H1 and ICOS in both tumor cells and TIL is associated with the metastasis of colorectal carcinoma. A recent study on B7 family molecules in tumor biological behavior demonstrated that aberrant expression of B7H1 in renal cell carcinoma apparently impairs T cell function and survival[13]. Our results and these data provide the morphological and clinical support to elucidate the role of B7 co-stimulatory molecules in tumor immune evasion.
The present findings indicate that new members of B7 family such as B7H1, B7H2 and ICOS are involved in promoting TH2- based responses preferentially. More interestingly, it has been reported that tumor-associated B7H1 can promote apoptosis of antigen -specific human T-cell clones in vitro[14,15], and mouse P815 tumor -expressed B7H1 increases apoptosis of activated tumor-reactive T- cells and promotes the growth of highly immunogenic B tumor in vivo[10]. These results suggest that induction of apoptosis of tumor-infiltrating lymphocytes by B7H1 molecules overexpressed in human colorectal carcinoma may be a potentially active escape strategy of various tumors from immune attack as is shown by Wintterle et al[16].
Tumor escape is attributed to a variety of immune evasion strategies, including downregulation of MHC-I class molecules, regulatory T cells, Th2 cytokines, secretion of immunosuppressive factors from tumor cells or TIL, and lack of T-cell co-stimulation. Tumor local microenvironment plays an essential role in determining the final destiny of antitumor immunity[17].
The discovery of new members of B7 family presenting different co-stimulatory effects indicates that different co-signaling molecules lead to different results, such as enhancement of T-cell Receptor (TCR) -mediated immune responses or inhibition of TCR-mediated immune responses. Tumor -associated B7H1 might produce tumor immune escape by promoting apoptosis of tumor-reactive T-cells and type II cytokine secretion through PD-1 ligand[18,19]. Hirano et al[20] reported that B7H1 /PD-1 forms a molecular shield to prevent destruction by CTL, suggesting that to block B7H1 or PD- 1 by specific monoclonal antibodies could reverse this resistance and profoundly enhance therapeutic efficacy. Strome et al[21] have shown the feasibility of the new immunotherapy. With the suggestion of the concept of co-inhibitors[22], investigation of the functional characteristics of tumor-associated signal molecules may contribute to exploitation of T cell-based tumor immunotherapy.
| 1. | Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellström I, Hellström KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 352] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 517] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 3. | Si LS, Chen YY, Wang YL, Guo JF, Sun Y. B7-1 Molecule ex-pression on tumor cells in human cancerous tissues. Chin Med Sci J. 1998;13:195-198. |
| 4. | Guo J, Si L, Wang Y. An in situ study on immunostimulatory molecules in cancer cells within the cervical carcinoma tissues. Zhonghua YiXue ZaZhi. 2000;80:342-345. [PubMed] |
| 5. | Liu P, Xiao JX, Li R, Chen XL, Lai BC, Si LS, Wang YL. Analysis on local immune environment of human gastric car-cinoma in situ. J Tumor Marker Oncol. 2003;18:80-85. |
| 6. | Li R, Rüttinger D, Li R, Si LS, Wang YL. Analysis of the immunological microenvironment at the tumor site in patients with non-small cell lung cancer. Langenbecks Arch Surg. 2003;388:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2001] [Article Influence: 74.1] [Reference Citation Analysis (5)] |
| 8. | Watanabe M, Watanabe S, Hara Y, Harada Y, Kubo M, Tanabe K, Toma H, Abe R. ICOS-mediated costimulation on Th2 differentiation is achieved by the enhancement of IL-4 receptor-mediated signaling. J Immunol. 2005;174:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 719] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170:2310-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Fujiwara K, Higashi T, Nouso K, Nakatsukasa H, Kobayashi Y, Uemura M, Nakamura S, Sato S, Hanafusa T, Yumoto Y. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2004;19:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Ke X, Jia L, Wang J, Wang D. Transfection of B7-1 cDNA empowers antigen presentation of blood malignant cells for activation of anti-tumor T cells. Chin Med J (Engl). 2003;116:78-84. [PubMed] |
| 13. | Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174-17179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 650] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 658] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 15. | Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med (Berl). 2003;81:281-287. [PubMed] |
| 16. | Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462-7467. [PubMed] |
| 17. | Chu Y, Hu HM, Winter H, Wood WJ, Doran T, Lashley D, Bashey J, Schuster J, Wood J, Lowe BA. Examining the immune response in sentinel lymph nodes of mice and men. Eur J Nucl Med. 1999;26:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 471] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 19. | Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1: PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089-1096. [PubMed] |
| 21. | Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501-6505. [PubMed] |
| 22. | Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 1004] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK