INTRODUCTION
Acute esophageal necrosis (AEN) in the absence of ingestion of a caustic or corrosive agent has been reported to be extremely rare. AEN is defined as a dark pigmentation of the esophagus associated with histologic mucosal necrosis. The exact pathogenesis is still unknown, but several etiologies have been suggested including ischemia[1,2], gastric outlet obstruction[3], hypersensitivity to antibiotics[4], gastric volvulus[5], and viral infection[6]. We reported a case of AEN caused by alcohol abuse.
CASE REPORT
A 41-year-old man visited the emergency room with a 12-h history of epigastric pain, violent vomiting, and coffee-ground emesis. His past medical history included alcoholic liver injury. He had felt nausea and appetite loss for a week. The previous day, he had consumed 1.8 L of shochu, distilled spirits containing 25% alcohol. He denied the ingestion of any caustic agents, antibiotics, and NSAIDs. He was afebrile with a regular pulse of 110 bpm, a blood pressure of 74/40 mmHg, a respiratory rate of 26 breaths/min and a core temperature of 37.0 °C. Physical examination revealed a mild distended abdomen and epigastric tenderness. The liver edge was measured at 4 cm below the right costal margin. A Foley catheter was inserted into the bladder, but no urine was returned. Arterial blood gas measurements using with O2 flow at 3 L/min was: pH 6.981, PaCO2 16.4 mmHg, PaO2 156.4 mmHg, AG 55.7 mmol/L, HCO3 3.9 mmol/L, lactate 26.4 mmol/L. Laboratory data were: WBC 32 430/mm3, hemoglobin 149 g/L, platelets 194 K/mm3, TP 55 g/L (normal 67-83 g/L), SGOT 169 U/L (8-38 U/L), SGPT 47 U/L (4-44 U/L), T. chol 2 670 mg/L (1 270-2 380 mg/L), TG 20 370 mg/L (430-1 610 mg/L), BUN 320 mg/L (80-200 mg/L), creatinine 33 mg/L (6-10 mg/L), Na 129 mmol/L (138-149 mmol/L), K 5.4 mmol/L (3.5-5.0 mmol/L), Cl 71 mmol/L (99-110 mmol/L), Ca 65 mg/L (82-102 mg/L), amylase 484 U/L (43-116 U/L). Abdominal CT demonstrated a grossly dilated stomach, a few linear strands in peripancreatic fat suggesting inflammation, a small amount of fluid in the anterior pararenal space, a thickened distal esophagus with evidence of transmural inflammation, but no free air. A naso-gastric tube was placed and 800 mL of brown liquid was removed. The patient was admitted and received intravenous fluids with 80 mL of 7% sodium bicarbonate. He was rapidly rehydrated and urinalysis did not reveal ketones. Arterial blood gas, 5 h after his admission, showed pH 7.505, lactate 4.1 mmol/L.
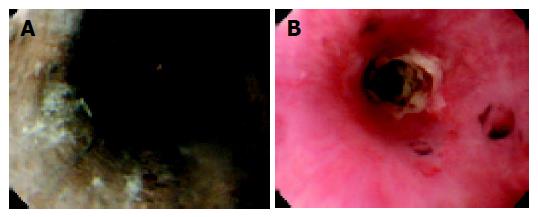
Esophago-gastro-duodenoscopy (EGD), on the next day, demonstrated black and friable mucosa from 3 to 4 cm below the cricopharyngeus to the cardia (Figure 1A). Biopsies of the esophagus were not taken at this time due to concern over possible perforation. The stomach had a few linear ulcers and the mucosa was edematous. A barium swallow revealed thickened esophagus and decreased esophageal motility, but no evidence of perforation.
Figure 1 A: Endoscopy on d 2 shows that esophagus is circumferentially black and friable.
B: Endoscopy on d 24 shows stricture formation at the middle esophagus. The mucosa is covered by white exudate.
Platelets count was rapidly decreased (d 3: 74 K/mm3) and we considered disseminated intravascular coagulation. Treatment was conservative management with oral intake restriction, intermittent naso-gastric tube suction, total parental nutrition, intravenous famotidine, antibiotics, and nafamostat mesilate. Laboratory data, including thrombocytopenia, were gradually recovered.
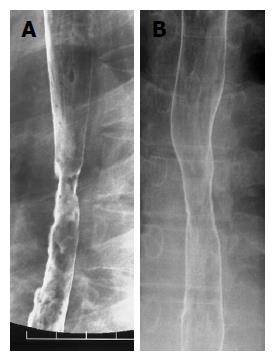
Ten days after admission, EGD revealed the presence of a thick, white exudate on the pink esophageal mucosa. Oral feeding was started on d 13. However, he sometimes complained about chest oppression after meal. Both an EGD on d 24 (Figure 1B) and a barium swallow on d 27 (Figure 2A) demonstrated stricture of the middle esophagus. On d 39, we performed balloon dilatation to the stricture. With the assistance of esophagoscope and a guidewire, a 20-mm balloon dilator was inserted to the stricture. The balloon dilator was inflated to a pressure of 2 atm for 5 min. Balloon dilatation was accomplished without any complication. Two days after, the barium swallow demonstrated improvement of the stricture (Figure 2B). The patient was discharged without complaints on d 46 taking 20 mg of omeprazole once a day. His alcoholic hepatitis was reversible and laboratory data has been normal since discharge.
Figure 2 Barium swallow study.
A: Stricture formation at middle esophagus on d 27. The diameter of the lumen was 6 mm; B: On d 41, improvement of the stricture was seen after balloon dilatation.
DISCUSSION
AEN or Black esophagus was first described in 1990 by Goldenberg et al[1], although two post-mortem cases had previously been reported[7,8]. The precise incidence and prevalence of this disease are unknown. Autopsy studies have estimated its prevalence at 0.1-0.2%[9,10]. Moreto et al[11], have identified 10 cases of AEN in a review of more than 80 000 upper endoscopies, an incidence rate of only 0.0125%. Among variety of mechanisms for the development of this condition including direct toxic effect[12,13] or indirect acidic effect[14,15], low systemic perfusion seems to play the dominant role. The main complications are esophageal stenosis and stricture formation[3].
Lactic acidosis is the cause of sudden death in alcoholics[16]. It is caused by an elevation of the NADH/NAD+ ratio with increased formation of lactate from pyruvate, diminished gluconeogenesis and decreased lactate uptake into the liver[17,18]. Thiamine deficiency due to malnutrition and the direct action of alcohol are responsible for the occurrence of lactic acidosis[19,20]. Five typical symptoms of alcoholic lactic acidosis are vomiting, nausea, abdominal pain, tachycardia and the Kussmaul form of respiration. In this case, we suspect that his acidosis appeared at least a week before his admission because his nausea and appetite loss had started. Lactic acidosis might have been worsened by his malnutrition and alcohol abuse.
The fall in blood pH had profound effects on circulation systems. Intrinsic cardiac contractility may be depressed, but inotropic function can be normal because of catecholamine release. Both peripheral arterial vasodilatation and central venoconstruction can be present. Several reports described cases of stroke[21], intestinal necrosis[22], heart failure, and pancreatitis[23] in severe metabolic acidosis and those may be associated with physiologic response to hypovolemia and subsequent poor organ perfusion caused by severe acidosis. Low esophageal perfusion pressure, a presumed cause of our case, was suggested by his original hypotension. In addition, coagulation abnormality by acute pancreatitis can aggravate esophageal mucosal perfusion. Severe peripheral circulatory failure also makes massive gastric distension and renal insufficiency. Moreover, alcohol overindulgence induces irritation of the gastric mucosa and the accumulation of large volume of gastric secretions. This can overwhelm the normal defense of the esophagus, and could be another cause of our case.
In conclusion, we reported a case of AEN caused by alcohol abuse. The main pathogenesis can be accounted for low systemic perfusion caused by severe lactic acidosis. The stricture area of middle esophagus was managed with balloon dilatation.