Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5475
Revised: July 23, 2004
Accepted: July 27, 2004
Published online: September 21, 2005
AIM: To establish the pig model of pancreatoduodenal transplantation with enteric drainage (ED) and portal venous drainage (PVD).
METHODS: Forty-six hybrid Landrace pigs were divided into two groups (donors and recipients) randomly, and pancreatoduodenal allotransplantation was performed. Donors were perfused via abdominal aorta without clamping the portal venous outflow with UW solution at 80100 cm H2O after heparinization. Whole pancreato-duodenal grafts were harvested with segments of abdominal aorta and portal vein, and shaped under 4 °C UW solution. Then, end-to-end anastomosis was performed with the donor iliac artery bifurcation Y graft to the recipient superior mesenteric artery and celiac artery. Furthermore, type I diabetes model was made by removal of the recipient pancreas. The venous anastomosis was reconstructed between the donor portal vein and the recipient superior mesentery vein. Meanwhile, end-to-side anastomosis was performed with the donor common iliac artery bifurcation Y graft to the recipient abdominal aorta, and side-to-side intestinal anastomosis was performed between the donor duodenum and the recipient jejunum. External jugular vein was intubated for transfusion. Levels of plasma glucose, insulin and glucagon were measured during the operation and on the 1st, 3rd, 5th, and 7th d after operation.
RESULTS: Pancreatoduodenal allotransplantation was performed on 23 pigs of which 1 died of complication of anesthesia. The success rate of operation was 95.6%. Complications of operation occurred in two cases in which one was phlebothrombosis with an incidence of 4.6%, and the other was duodenojejunal anastomotic leak with an incidence of 4.6%. The level of plasma glucose decreased within 30 min, after removal of pancreas and recovered on the 2nd d after operation. The level of plasma insulin and glucagon increased within 30 min after removal of pancreas and recovered on the 2nd d after operation. Rejection occurred on the 1st d and reached the worst level on the 7th d after transplantation, without change of plasma insulin and glucagon or clinical symptoms of rejection.
CONCLUSION: Pancreatoduodenal transplantation in pigs can treat type I diabetes. ED and PVD can keep the function of endocrine in normal. The technique of pancreatoduodenal transplantation with ED and PVD may pave the way for the further application of pancreas transplantation in clinic.
- Citation: Zhang ZD, Han FH, Meng LX. Establishment of a pig model with enteric and portal venous drainage of pancreatoduodenal transplantation. World J Gastroenterol 2005; 11(35): 5475-5479
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5475
Pancreas transplantation or combined pancreas-kidney transplantation is a radical therapy method for insulin-dependent diabetes mellitus and its complications. Though immune inhibitors and improvement of surgical technology have achieved obvious improvement of graft functional survival, surgical technology is still one of the important factors contributing to the failure of pancreas transplantation. The rate of it was 7-14% to simultaneous pancreas-kidney transplantation (SPK), 8-23% to pancreas after kidney transplantation (PAK), 10-23% to pancreas transplantation alone (PTA). Since 1995, over 1 000 cases of pancreas transplantation have been done[1]. Surgeons always want to decrease the failure caused by surgical technology, and to perform pancreas transplantation according to the status of anatomy and physiology. To pancreas transplantation, there are two types of exocrine drainage, one is bladder drainage (BD), the other is enteric drainage (ED). There are two types of endocrine drainage, one is systemic drainage (SVD), the other is portal venous drainage (PVD). BD could cause metabolic acidosis, uterine reflux pancreatitis, and urinary bladder stimulation that contributes to mucous erosion, bleeding, urinary tract stricture, etc.; so ED has to be performed after about 25% of BD operations[2]. In SVD cases, insulin and glucagon flow into systemic venous system, not through the liver first; so it is not good in improving of microvascular lesions[3]. In PVD cases, pancreas glucagonoma can be avoided, and immune intolerance can be induced because the graft antigen goes into the liver directly. The combination of ED and PVD in pancreas transplantation is better to the status of anatomy and physiology. The pig anatomy structure is similar to that of human being, so it is very useful to set up an animal model with combined ED and PVD in pancreas transplantation.
Forty-six local Sichuan hybrid Landrace pigs, weighing 25-32 kg, were used as donors and recipients, and were provided by the Pig Institute of Sichuan Agriculture Science Academy. They were fasted for 12 h and forbidden drinking water for 6 h before operation.
Atropine (0.5 mg) and ketamine (15-20 mg/kg) were intramuscularly injected before anesthesia, 0.25% thiopental sodium was intravenously used, ventilation was provided by tracheal intubation. The anesthesia was maintained with 2% procaine and 0.15% succinylcholine chloride. During operation, ketamine, diazepam or fentanyl was sometimes used for better anesthesia; ECG, arterial pressure and SaO2 were monitored. A trocar was placed in the auricular vein for fluid infusion.
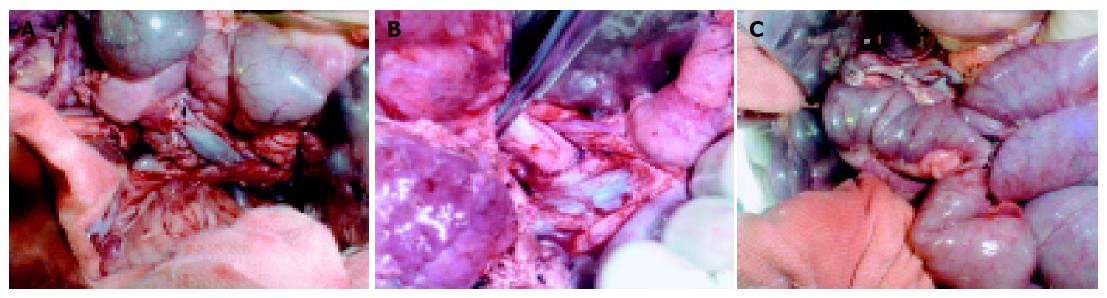
We used anterior abdominal median incision. The stomach was removed, and the end of duodenum was blocked. The proper hepatic artery and common bile duct were ligated and divided. The portal vein was isolated. The superior mesentery artery and vein were isolated and ligated. The jejunum was divided at the site of 5 cm to the superior mesentery artery, the bilateral renal arteries and veins were isolated and ligated. The spleen artery and vein were ligated. Heparin (200 IU/kg) was intravenously injected to get systemic heparinization, 1 000 mL of normal solution (4 °C) was poured into abdominal cavity. An abdominal aorta cannula was placed, the abdominal aorta was blocked at the site up to the celiac artery, and the in site flushing of fluctuating UW (4 °C) fluid (about 2 000 mL) was made through the former cannula. The perfusion pressure was 80-100 cm water column. The portal vein was divided, and the end near the pancreas was open, the other end was ligated. The perfusion was stopped when the temperature was down, pancreas became pale, and effluent from the portal vein was clear (Figure 1). The head of pancreas was mobilized to the right of aorta, the tail of pancreas and spleen were mobilized. The aorta was divided above its fork, lumbar arteries derived from the proximal part of abdominal aorta were ligated and divided. The left or right common iliac artery and internal and external iliac arteries were removed.
The pancreas and duodenum were stored in UW fluid (4 °C) of a plastic bag, which was placed in a basin containing cold normal solution (Figure 2). The abdominal aorta was opened in the back wall, the peripheral of celiac artery and superior mesentery artery remained as elliptic Carrel patch, whose diameters were 0.5-0.8 cm. The celiac stem cannula and superior mesenteric artery cannula were placed for UW fluid perfusion. The portal vein was modified and 1.5-3.0 cm of it remained. The small blood and lymphatic vessels around the pancreas were ligated one by one. The fat tissue and lymph nodes were removed. The incision of anterior duodenum wall was made for the wash with metronidazole (0.5 g) and amikacin (0.4 g). The length of 20-30-cm duodenum was left for transplantation. An anastomosis was made for the opening of celiac stem and the distal end of external iliac artery, so did for the opening of superior mesenteric artery and the distal end of internal iliac artery. The modified graft was placed in UW fluid (4 °C).
After anesthesia worked, a cannula was placed in the external jugular vein, an anterior abdominal median incision (18-20 cm) was applied. Then the whole pancreas was removed. The superior mesentery vein and portal vein were exposed. After the anterior part of superior mesentery vein or portal vein was clamped with a vessel nip, whose caliber was similar to the diameter of the proximal end of the donor portal vein, was made. The lacuna was rinsed with normal saline containing heparin. The donor portal vein anastomosis was performed in an end-to-side fashion to the recipient superior mesenteric vein or portal vein (Figure 3A). The abdominal aorta distal to the renal vein was freed. After the anterior part of abdominal aorta was clamped with a Satinski nip, an incision (1.0-1.5 cm) was made in corresponding part of the recipient anterior abdominal aorta wall. The donor common iliac artery anastomosis was performed in an end-to-side fashion to the recipient aorta (Figure 3B). The blood circulation to the graft was recovered after the former anastomoses were finished, the graft became pink, and its arteriopalmus and peristalsis were restored. The donor duodenum anastomosis was performed in a side-to-side fashion to the recipient jejunum 8-10 cm distal to the recipient Treitz ligament (Figure 3C). After the spleen was removed, the graft was placed in the right part of abdominal cavity.
After autonomous respiration, steady blood pressure, and normal pulse were recovered, the tracheal intubate was removed. After operation, the recipient pig was forbidden drinking for 24 h, and eating for 2-4 d, and received 1 000-2 500 mL fluid infusion intravenously in those days. During the 1st d after operation, the recipient received the fluid at the ratio of glucose to insulin 1:4, 3 g ampicillin, 0.5 g of metronidazole, 40 mL danshen injection, 500 mL low molecular dextran, and heparin (1 mg/kg). To monitor the graft function status, the plasma glucose, insulin, and glucagon were measured, and pathology examination was performed. Plasma glucose, insulin, and glucagon were measured 30 min after the pancreas was removed, 30 min after blood circulation to the graft was recovered, and on each of the first 7 d after operation. The graft biopsy tissues were obtained on the 1st, 3rd, 5th and 7th d after transplantation. The biopsy tissues were observed by naked eyes and microscope after being stained with HE. The Nakhleh criteria[4] were used as follows:
Criteria for pancreas rejection The score was 0, if no rejection could be seen. The score was 1, if mild rejection could be seen (Lymph cells could be seen scattering in external secretion tissues, especially in tissues among pancreas lobules. The pancreas islet and pancreas duct were normal.). The score was 2, if moderate rejection could be seen (Diffused lymph cells and plasma cells can be seen in pancreas tissue, and local necrosis lesions or small pitch of wrecked granules could be seen. Lymph cells infiltrated in the area around pancreas duct. The structure of islet was normal, sometimes a few inflammation cells could be seen in islet.). The score was 3, if severe rejection could be seen (Much more lymph cells and plasma cells can be seen in pancreas tissue. Sweeping wreck of granules and sometimes pancreas fibrosis could be seen. Islets were wrecked and some or all of them disappeared. Vascular rejection, vessel endothelium inflammation, and fibroid necrosis could be seen.).
Criteria for vascular rejection The score was 1, if lymph cells infiltrated into the area under minority of venous endothelium cells. The score was 2, if lymph cells infiltrated into the area under majority of venous endothelium cells. The score was 3, if lymph cell infiltration and inflammation around the veins could be seen.
SPSS10.0 was used for analysis of data.
Of the 23 pigs that received pancreas transplantation, only one pig died from anesthesia accident. The survival rate was 95.65%. The longest survival time was 96 d. The operation time of donors was 73.84±6.86 min, and that of recipients was 143.25±18.14 min. There was no warm ischemia, the time of cold ischemia was 117.30±18.02 min. The portal vein anastomosis was 38.305.60 min, the time of artery anastomosis was 26.20±2.26 min. Two complications were observed, one was pancreas phlebothrombosis, the other was duodenojejunal anastomotic leak. There was no complication of vessel anastomosis, transplantational pancreatitis, or pancreatic ascites.
The graft biopsy tissues were obtained on the 1st, 3rd, 5th, and 7th d after transplantation. There was one case of pancreas infarction. Seventeen cases survived with good blood supply to pancreas.
Normal limosis plasma glucose of pigs was 5.22±0.92 mmol/L, plasma insulin was 8.34±2.43 mU/L, plasma glucagon was 177.73±12.11 pg/mL.
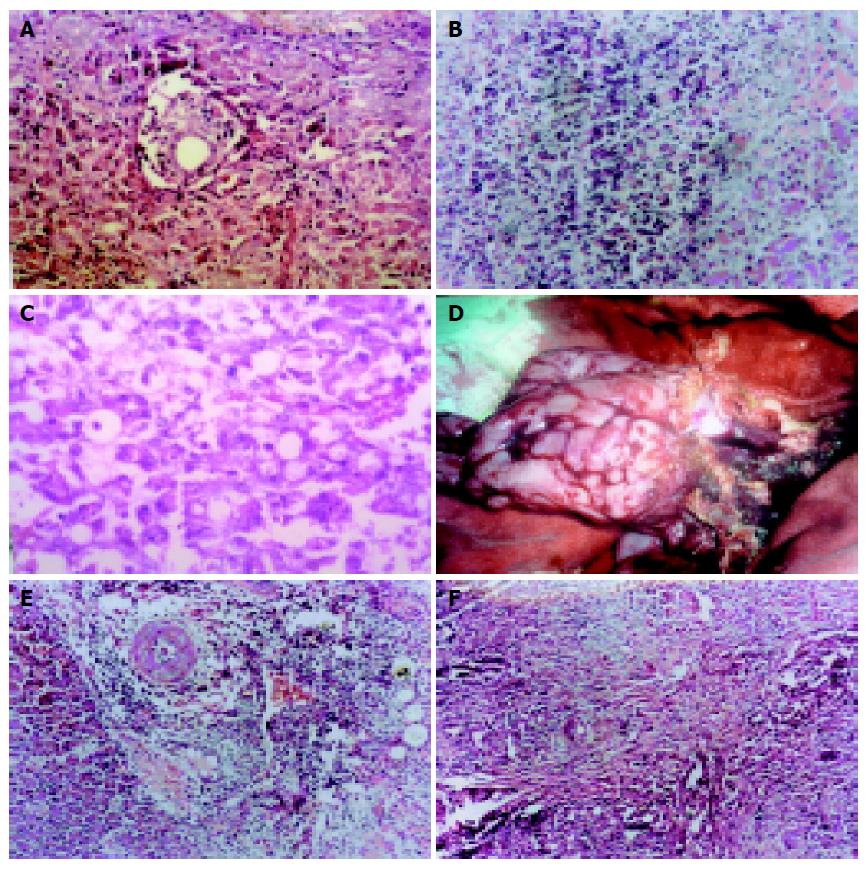
During the first 3 d after transplantation, mild edema of pancreas could be seen. There were no exudation, congestion, or adhesion of pancreas. Under microscopy, lymph cells and monocytes could be seen in pancreas matrix, not in islet (Figure 4A). During the 5th-7th d, there were mild edema, congestion and adhesion of pancreas. Under microscopy, local or extensive infiltration of lymph cells and monocytes, apoptosis body could be seen (Figures 4B and C). There was separated or small pitch of necrosis of pancreas adrenal cells, extensive infiltration of lymph cells could be seen around small vessels, a few lymph cells could be seen around islets. During the 7th-9th d, the pancreas became more flexible, adhered to peripheral tissues, looked pale, and had good blood supply (Figure 4D). Under microscopy, extensive infiltration of lymph cells and monocytes could be seen in pancreas and around vessels. There were vessel endothelium inflammation and extensive fibrosis of pancreas (Figure 4E). During the 2nd-4th wk after operation, the pancreas glands and islets were replaced by connective tissues. Extensive lymphocyte infiltration could be seen in pancreas (Figure 4F).
Our data indicated that the plasma glucose arose 30 min after the pancreas was removed, became normal 2 d after transplantation, and arose again 15-21 d after transplantation. The half life of insulin or glucagon was only 5-10 min, indicating that the graft endocrine function was normal 2 d after transplantation. There was no host plasma insulin or glucagon after the recovery of grafts. It was different from SVD, the plasma insulin and glucagon levels were normal[5,6] indicating that the PVD in pancreas transplantation is better for the treatment of type I diabetes mellitus. To the rejection, our data indicated that mild rejection appeared on the 1st d after operation, and became more serious with the time protracted. At the same time (2 wk after transplantation) plasma glucose, insulin, and glucagon were normal, indicating that we cannot know if there is rejection, or how serious the rejection is, after the operation. More sensitive criterion must be found for clinical work[7]. The length and diameter of pig portal vein are similar to those of human beings[8], setting up of a pig animal model is beneficial in clinical pancreas transplantation with ED+PVD. It was reported that primary ED was associated with a high incidence of early graft thrombosis, a complication that is the major cause of graft loss in PTA recipients[9]. Our data show that only 1/23 had thrombosis of pancreas, no complication of vessel anastomosis was found.
Our pig model of pancreas transplantation was improved on the following aspects[5,10,11]: (1) The outflow of the graft was performed through the anastomosis of donor portal vein to the recipient inferior vena cava. (2) The arterial inflow to the allograft was achieved by a single anastomosis of an aortic patch to the intended target in the recipient. (3) The pancreas exocrine drainage to the urinary bladder was performed through the duodenocystostomy. The present technique has the following advantages. (1) The outflow of the graft was performed through the anastomosis of donor portal vein to the recipient portal vein. It is better to the status of anatomy and physiology. (2) The Y-graft was anastomosed to the recipient abdominal aorta and the blood supply was achieved through it. It is a standard arterial reconstruction of clinical pancreas transplantation[12], because it is good for simultaneous liver procurrent[13]. The allograft was modified, and Y-graft was achieved under the protection of cold preservation solution. It did not prolong warm ischemia[14,15]. (3) The pancreas exocrine drainage to the jejunum was gained through an anastomosis of donor duodenum to the recipient jejunum. So there were no complications in duodenocystostomy, such as metabolic acidosis, uterine reflux pancreatitis, and urinary bladder stimulation that could contribute to mucous erosion, bleeding, urinary tract stricture, etc.[16]. (4) During the operation of blood vessel anastomosis, the abdominal aorta or inferior vena cava was not obstructed completely. Then, during the operation, the disadvantage effect on the recipient blood circulation was lesser.
In conclusion, our pig model is a model with ED and PVD. It is better to the status of anatomy and physiology. Now, more and more clinical pancreas transplantations with ED and PVD are performed[16,17], our pig model of pancreas transplantation can work better for the further study of pancreas transplantation.
| 1. | Sutherland DE, Cecka M, Gruessner AC. Report from the International Pancreas Transplant Registry--1998. Transplant Proc. 1999;31:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Weir GC, Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes. 1997;46:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | West M, Gruessner AC, Metrakos P, Sutherland DE, Gruessner RW. Conversion from bladder to enteric drainage after pancreaticoduodenal transplantations. Surgery. 1998;124:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Nakhleh RE, Sutherland DE, Tzardis P, Schechner R, Gruessner RW. Correlation of rejection of the duodenum with rejection of the pancreas in a pig model of pancreaticoduodenal transplantation. Transplantation. 1993;56:1353-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Xu ZK, Liu XL, Zhang W, Miao Y, Du JH. Establishment of a pig model of combined pancreas-kidney transplantation. World J Gastroenterol. 1999;5:172-174. [PubMed] |
| 6. | Katz H, Homan M, Velosa J, Robertson P, Rizza R. Effects of pancreas transplantation on postprandial glucose metabolism. N Engl J Med. 1991;325:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Fernandez JA, Claver MA, Llorente S, Gimeno L, Robles R, Ramirez P, Bueno FS, Rodriguez JM, Lujan JA, Munitiz V. Clinical noninvasive evaluation of simultaneous pancreas-kidney transplants with the combined use of gamma graphy, Doppler ultrasound and serum markers. Transplant Proc. 2002;34:209-211. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | Terajima H, Shirakata Y, Yagi T, Mashima S, Shinohara H, Satoh S, Arima Y, Gomi T, Hirose T, Takahashi R. Successful longterm xenoperfusion of the pig liver: continuous administration of prostaglandin EI and insulin. Transplantation. 1997;63:507-512. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sutherland DE, Gruessner A. Pancreas transplantation in the United States as reported to the United Network for Organ Sharing (UNOS) and analyzed by the International Pancreas Transplant Registry. Clin Transpl. 1995;9:49-67. |
| 10. | Gänger KH, Mettler D, Böss HP, Ruchti C, Stoffel M, Schilt W. Experimental duodeno-pancreatico-renal composite transplantation: a new alternative to avoid vascular thrombosis? Transplant Proc. 1987;19:3960-3964. [PubMed] |
| 11. | Gruessner RW, Nakhleh R, Tzardis P, Schechner R, Platt JL, Gruessner A, Tomadze G, Najarian JS, Sutherland DE. Differences in rejection grading after simultaneous pancreas and kidney transplantation in pigs. Transplantation. 1994;57:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sollinger HW, Odorico JS, Knechtle SJ, D'Alessandro AM, Kalayoglu M, Pirsch JD. Experience with 500 simultaneous pancreas-kidney transplants. Ann Surg. 1998;228:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Troppmann C, Gruessner AC, Benedetti E, Papalois BE, Dunn DL, Najarian JS, Sutherland DE, Gruessner RW. Vascular graft thrombosis after pancreatic transplantation: univariate and multivariate operative and nonoperative risk factor analysis. J Am Coll Surg. 1996;182:285-316. [PubMed] |
| 14. | Johnson CP, Roza AM, Adams MB. Simultaneous liver and pancreas procurement--a simplified method. Transplant Proc. 1990;22:425-426. [PubMed] |
| 15. | Mizrahi S, Boudreaux JP, Hayes DH, Hussey JL. Modified vascular reconstruction for pancreaticoduodenal allograft. Surg Gynecol Obstet. 1993;177:89-90. [PubMed] |
| 16. | Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 428] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Gruessner AC, Sutherland DE. Report for the international pancreas transplant registry-2000. Transplant Proc. 2001;33:1643-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Li WZ Language Editor Elsevier HK