Published online Sep 7, 2005. doi: 10.3748/wjg.v11.i33.5193
Revised: February 23, 2005
Accepted: February 26, 2005
Published online: September 7, 2005
AIM: To identify the trend, possible risk factors and any pattern change of hepatocellular carcinoma (HCC) in Egypt over a decade.
METHODS: All HCC patients attending Cairo Liver Center between January 1993 and December 2002, were enrolled in the study. Diagnosis of HCC was based on histopathological examination and/or detection of hepatic focal lesions by two imaging techniques plus α-fetoprotein level above 200 ng/mL. The duration of the study was divided into two periods of 5 years each; period I (1993-1997) and period II (1998-2002). Trend, demographic features of patients (age, gender, and residence), risk factors (HBsAg, HCV-Ab, schistosomiasis and others) and pattern of the focal lesions were compared between the two periods. Logistic regression model was fitted to calculate the adjusted odds ratios for the potential risk factors. The population attributable risk percentage was calculated to estimate the proportion of HCC attributed to hepatitis B and C viral infections.
RESULTS: Over a decade, 1 328 HCC patients out of 22 450 chronic liver disease (CLD) patients were diagnosed with an overall proportion of 5.9%. The annual proportion of HCC showed a significant rising trend from 4.0% in 1993 to 7.2% in 2002 (P = 0.000). A significant increase in male proportion from 82.5% to 87.6% (P = 0.009); M/F from 5:1 to 7:1 and a slight increase of the predominant age group (40-59 years) from 62.6% to 66.8% (P = 0.387) in periods I and II respectively, reflecting a shift to younger age group. In the bivariate analysis, HCC was significantly higher in rural residents, patients with history of schistoso-miasis and/or blood transfusion. Yet, after adjustment, these variables did not have a significant risk for development of HCC. There was a significant decline of HBsAg from 38.6% to 20.5% (P = 0.000), and a slight increase of HCV-Ab from 85.6% to 87.9% in periods I and II respectively. HBV conferred a higher risk to develop HCC more than HCV in period I (OR 1.9 vs 1.6) and period II (OR 2.7 vs 2.0), but the relative contribution of HBV for development of HCC declined in period II compared to period I (PAR% 4.2%, 21.32%). At presentation, diagnostic α-fetoprotein level (≥200 ng/mL) was demonstrated in 15.6% vs 28.9% and small HCC (≤3 cm) represented 14.9% vs 22.7% (P = 0.0002) in periods I and II respectively.
CONCLUSION: Over a decade, there was nearly a twofold increase of the proportion of HCC among CLD patients in Egypt with a significant decline of HBV and slight increase of HCV as risk factors. α-Fetoprotein played a limited role in diagnosis of HCC, compared to imaging techniques. Increased detection of small lesions at presentation reflects increased awareness of the condition.
- Citation: El-Zayadi AR, Badran HM, Barakat EM, Attia MED, Shawky S, Mohamed MK, Selim O, Saeid A. Hepatocellular carcinoma in Egypt: A single center study over a decade. World J Gastroenterol 2005; 11(33): 5193-5198
- URL: https://www.wjgnet.com/1007-9327/full/v11/i33/5193.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i33.5193
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in the world[1] complicating liver cirrhosis in most cases. Its incidence is increasing worldwide ranging between 3% and 9% annually[2]. In Egypt, HCC was reported to account for about 4.7% of chronic liver disease (CLD) patients[3]. The epidemiology of HCC is characterized by marked demographic and geographic variations.
HBV is considered as a major risk factor for the progression to liver cirrhosis and HCC[4]. The relative risk of developing HCC for HBV carriers may be 100-200-fold higher than that for non-carriers[5]. Integration of the viral DNA into host genome was suggested to be the initiating event for HBV-induced carcinogenesis[6]. A recent study suggests that the HBx protein may inactivate p53 (tumor suppressor gene) leading to development of HCC[7]. However, the prevalence of HBV infection in Egypt has been declining over the last two decades[3].
The rising trend of HCC has been associated with increased prevalence of hepatitis C virus (HCV) infection[1]. In Egypt, the prevalence of HCV infection among general population has been estimated to be around 14%[8]. The fundamental mechanism by which HCV is related to HCC is not definitely known. Several lines of evidence indicate a strong causal association between HCV and HCC, as shown by the raised prevalence of anti-HCV[2,3] and/or HCV-RNA[9] in patients with HCC.
HCV mostly plays an indirect role in tumor development and appears to increase the risk of HCC by promoting fibrosis and cirrhosis. On the other hand, HCV may play a direct role in hepatic carcinogenesis through involvement of viral gene products in inducing liver cell proliferation[10]. However, it seems that cirrhosis is the common pathway by which several risk factors exert their carcinogenic effect[11].
Exposure to aflatoxin is an additional risk factor for the development of HCC, through damage of DNA in liver cells and mutation in p53 tumor suppressor gene[7]. A previous study showed that aflatoxin B1 has a considerable role in the development of HCC among Egyptians[3].
The aim of this study was to detect the trend of HCC in Egypt over the past decade, identify the possible risk factors and the proportion attributed to hepatitis B and C viral infections and changes in HCC pattern.
The study was conducted at Cairo Liver Center (CLC), a private institute established to provide specialized care for patients with liver disease. The center receives patients from almost all regions of Egypt, besides patients from the neighboring Arab countries.
A specially designed database application was employed for collecting and managing data of the Egyptian patients attending the center during the period from January 1993 to December 2002. Patients were assigned unique records, which were presented through a designed form interface. The form consisted of three sections on socio-demographic characteristics (age, gender, occupation, and residence), history of exposure to known risk factors of viral hepatitis - blood transfusion and parenteral anti-schistosomal therapy, and laboratory findings (HBsAg status, HCV-Ab status, α-fetoprotein level). Another patient record-linked application was employed to record the imaging characteristics of HCC cases such as location, number, and size of hepatic focal lesions, as well as, portal vein status.
HCV-Ab was detected using second generation ELISA (Boehringer Mannheim Immunodiagnostics for ES-300). HBsAg was confirmed by ELISA test (ELISA-Abbot Laboratories), α-fetoprotein level was tested using Abbot Laboratories’ method. No study in Egypt has stated the cut-off level of α-fetoprotein in Egypt yet; however, we considered a cut-off level of 200 ng/mL as reported internationally[12]. Ultrasonography was performed using Toshiba Sonolayer 600, with a convex linear probe using B mode.
Diagnosis of HCC was based on histopathological examination and/or detection of hepatic focal lesions by two imaging techniques (ultrasonography and dynamic CT) plus α-fetoprotein level above 200 ng/mL.
Data analysis was done using SPSS computer package. Trend of HCC among CLD patients was studied annually and χ2 test for trend was used to detect significant annual differences. Also, the 10-year period was divided into period I from 1993 to 1997, and period 2 from 1998 to 2002. The differences in the characteristics of HCC patients between the two periods were compared. χ2 test for association was used to detect significant associations between proportions.
Logistic regression model was fitted to identify the impact of the various risk factors on HCC. The dependent outcome was HCC (0 = absent, 1 = present) and the independent factors were age, sex, previous history of blood transfusion, schistosomiasis, HCV-Ab positivity and HBsAg positivity.
The population attributable risk percent (PAR%)[13] was calculated to express the proportion of HCC in the study population that was attributed to HCV-Ab or HBsAg positivity and thus can be eliminated, if exposure is prevented. PAR% was calculated as follows:
PAR% = Pe(OR-1)/Pe(OR-1)+1
Where Pe is the proportion of exposed individuals in the population and OR represents the adjusted odds ratio.
A total of 22 450 Egyptian patients with CLDs were registered at CLC between the year 1993 and 2002 (Table 1). Their ages ranged from 33 to 74 years (mean±SD = 53.3±11.67 years). 77.7% were males and 22.3% females and most of them (75.2%) resided in rural areas. Around 50% had history of parenteral anti-schistosomal therapy and 12.9% had history of blood transfusion. HCV-Ab accounted for 72.3% and HBsAg for 20.4%.
| Total registered (n = 22 450) | HCC negative (n = 21 122) | HCC positive (n = 1 328) | ||||
| Age (P = 0.000) | ||||||
| <40 | 5 670 | 25.3 | 5 591 | 26.5 | 79 | 5.9 |
| 40-59 | 13 717 | 61.1 | 12 858 | 60.9 | 859 | 64.7 |
| 60+ | 3 063 | 13.6 | 2 673 | 12.7 | 390 | 29.4 |
| Sex (P = 0.000) | ||||||
| Men | 17 442 | 77.7 | 16 308 | 77.2 | 1 134 | 85.4 |
| Women | 5 008 | 22.3 | 4 814 | 22.8 | 194 | 14.6 |
| Residence (P = 0.000) | ||||||
| Rural | 16 882 | 75.2 | 15 805 | 74.8 | 1 078 | 81.2 |
| Urban | 5 568 | 24.8 | 5 317 | 25.2 | 250 | 18.8 |
| Blood transfusion (P = 0.004) | ||||||
| No | 19 548 | 87.1 | 18 426 | 87.2 | 1 122 | 84.5 |
| Yes | 2 902 | 12.9 | 2 696 | 12.8 | 206 | 15.5 |
| Schistosomiasis (P = 0.000) | ||||||
| No | 10 126 | 45.1 | 9 617 | 45.5 | 509 | 38.3 |
| Yes | 12 324 | 54.9 | 11 505 | 54.5 | 819 | 61.7 |
| HCV-Ab (P = 0.000) | ||||||
| Negative | 6 209 | 27.7 | 6 035 | 28.6 | 174 | 13.1 |
| Positive | 16 241 | 72.3 | 15 087 | 71.4 | 1 154 | 86.9 |
| HBsAg (P = 0.000) | ||||||
| Negative | 17 864 | 79.6 | 16 913 | 80.1 | 951 | 71.6 |
| Positive | 4 586 | 20.4 | 4 209 | 19.9 | 377 | 28.4 |
There were 1 328 HCC cases diagnosed over the 10-year period giving an overall proportion of 5.9%. HCC was diagnosed mainly among males between 40 and 59 years of age living in rural areas. Schistosomiasis, blood transfusion, HCV-Ab, and HBsAg were more prevalent among HCC-positive cases than HCC-negative cases (Table 1).
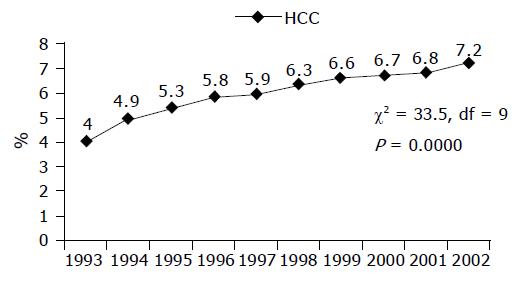
As shown in Table 2 and Figure 1, there was a significant annual increase (χ2 = 29.9, df = 9, P = 0.000) in the proportion of patients with HCC attending CLC, during the study period ranging from 4.0% in 1993 to 7.2% in 2002.
| Total registered | HCC | ||
| n | n | % | |
| 1993 | 2 636 | 106 | 4 |
| 1994 | 2 160 | 106 | 4.9 |
| 1995 | 2 054 | 109 | 5.3 |
| 1996 | 2 236 | 130 | 5.8 |
| 1997 | 2 154 | 127 | 5.9 |
| 1998 | 2 459 | 155 | 6.3 |
| 1999 | 2 448 | 162 | 6.6 |
| 2000 | 2 449 | 164 | 6.7 |
| 2001 | 2 064 | 140 | 6.8 |
| 2002 | 1 790 | 129 | 7.2 |
| Total | 22 450 | 1 328 | 5.9 |
Comparing the two periods (Table 3), there was a slight shift in age distribution to younger age group from age group ≥60 years, to the most predominant age group 40-59 years, but the difference did not reach a statistical significance (P = 0.387). There were no significant changes as regards residence and history of schistosomiasis between the two periods. However, there was a significant increase in the proportion of males (χ2 = 6.7, df = 1, P = 0.009) in period II (Table 3).
| 1993-1997 | 1998-2002 | P | |||
| HCC (n = 578) | HCC (n = 750) | ||||
| n | % | n | % | ||
| Age (yr) | 0.387 | ||||
| <40 | 36 | 6.2 | 43 | 5.7 | |
| 40-59 | 362 | 62.6 | 497 | 66.3 | |
| 60+ | 180 | 31.1 | 210 | 28 | |
| Sex | 0.009 | ||||
| Male | 477 | 82.5 | 657 | 87.6 | |
| Female | 101 | 17.5 | 93 | 12.4 | |
| Residence | 0.411 | ||||
| Rural | 475 | 82.2 | 603 | 80.4 | |
| Urban | 103 | 17.8 | 147 | 19.6 | |
| Schistosomiasis | 371 | 64.2 | 448 | 59.7 | 0.098 |
| Blood transfusion | 113 | 19.6 | 93 | 12.4 | 0.00 |
| HCV-Ab | 495 | 85.6 | 659 | 87.9 | 0.233 |
| HBsAg | 223 | 38.6 | 154 | 20.5 | 0.00 |
| Co-infection (HBV+HCV) | 156 | 27 | 81 | 10.8 | 0.00 |
| Non B Non C | 16 | 2.8 | 18 | 2.4 | 0.673 |
| α-Fetoprotein (≥200 ng/mL) | 90 | 15.6 | 217 | 28.9 | 0.00 |
| Number of lesions | 0.006 | ||||
| Unifocal | 264 | 45.7 | 286 | 38.1 | |
| Multifocal | 314 | 54.3 | 464 | 61.9 | |
| Site of lesion | |||||
| Right lobe | 339 | 58.7 | 524 | 69.9 | 0.00 |
| Left lobe | 73 | 12.6 | 105 | 14 | |
| Both | 166 | 28.7 | 121 | 16.1 | |
| Size of lesion | 0.00 | ||||
| 1≤3.0 cm | 86 | 14.9 | 170 | 22.7 | |
| >3.0 cm | 492 | 85.1 | 580 | 77.3 | |
| PVT2 | 104 | 18 | 134 | 17.9 | 0.953 |
Analysis of the major risk factors of HCC in period I compared to period II revealed appreciable changes (Table 3). There was a significant drop of HBsAg positivity from 38.6% to 20.5% (χ2 = 52.3, df = 1, P = 0.000), in hepatitis C and B co-infection from 27.0% to 10.8% (χ2 = 58.4, df = 1, P = 0.000) among HCC cases and decline in the proportion of patients with previous history of blood transfusion from 19.6% to 12.4% (χ2 = 12.7, df = 1 , P = 0.000). There was a slight increase of HCV-Ab positivity from 85.6% to 87.9% (χ2 = 1.42, df = 1, P = 0.23). Diagnostic α-fetoprotein level (≥200 ng/mL) was reported in 15.6% of patients in period I and 28.9% in period II (χ2 = 32.8, df = 1, P = 0.000, Table 3).
All HCC cases presented with focal hepatic lesions on imaging. The right lobe was affected in 65%, the left lobe in 13.4% and both lobes in 21.6%, most of which (54.9%) were diffusive or multinodular hepatic focal lesions. At presentation, the lesions tended to be multifocal (χ2 = 7.65, df = 1, P = 0.006), more on the right side (χ2 = 30.4, df = 1, P = 0.000) and large (>3 cm) in size (χ2 = 86.1, df = 2, P = 0.000). However, there was a significant increase in the detection of small lesions in the second period more than in the first period (χ2 = 12.7, df = 1, P = 0.000). Portal vein thrombosis was demonstrated in 18.0% of cases in period I and 17.9% in period II with no significant difference between the two periods (Table 3).
The adjusted odds ratios (Table 4) have shown that men were at higher risk to develop HCC than women (OR = 2.9) as well as patients of older age (age group 40-59 years, OR = 3.7 times and ≥60 years, OR = 11.2, P = 0.000). HCV-Ab-positive cases were at double risk, while HBsAg were at nearly triple risk of developing HCC. Residents of rural areas, patients with previous history of schistosomiasis and/or blood transfusion were significantly related to HCC in the bivariate analysis. However, after adjustment for other risk factors in the logistic regression model, their impact on acquiring HCC did not reach statistical significance. Although HBsAg occurrence conferred a higher risk of HCC than HCV in both periods, HBsAg appears to have a less share in HCC development as the PAR% revealed that 42.0% of HCC cases were attributed to HCV positivity, while only 23.4% of them were attributed to HBV positivity. HBsAg has a lower contribution for development of HCC in period II (PAR% = 4.2%) than in period I (PAR% = 21.3%).
| OR | 95%CI | P | PAR% | |
| Age (yr) | 0.0 | |||
| <40 | 1.0 | Reference | - | |
| 40-59 | 3.7 | 2.0-6.7 | - | |
| 60+ | 11.2 | 5.7-21.9 | - | |
| Sex | 0.0 | |||
| Men | 2.9 | 1.7-4.8 | - | |
| Women | 1.0 | Reference | - | |
| Residence | 0.654 | |||
| Rural | 1.0 | Reference | - | |
| Urban | 0.9 | 0.6-1.4 | - | |
| Blood transfusion | 0.908 | |||
| No | 1.0 | Reference | - | |
| Yes | 1.0 | 0.6-1.5 | - | |
| Schistosomiasis | 0.906 | |||
| No | 1.0 | Reference | - | |
| Yes | 1.1 | 0.7-1.5 | - | |
| HCV-Ab | 0.002 | |||
| Negative | 1.0 | Reference | - | |
| Positive | 2.0 | 1.3-3.0 | 42.0 | |
| HBsAg | 0.001 | |||
| Negative | 1.0 | Reference | - | |
| Positive | 2.5 | 1.4-4.4 | 23.4 |
Worldwide, HCC is one of the most common malignancies associated with poor prognosis[14]. According to recent reports, the incidence of HCC has increased sharply in the last 5-10 years[1,2,15]. In USA, the rate of HCC has increased by 70% over the last two decades[16]. Registry data in Canada and Western Europe show similar trends[16]. So far, no enough attention has been paid to explore the magnitude of the problem of HCC in Egypt. In the current study, there has been a remarkable increase of the proportion of HCC among CLD patients from 4.0% to 7.2% over a decade. This rising proportion may be explained by the increasing risk factors such as the emergence of HCV over the same period of time, the contribution of HBV infection, improvement of the screening programs and diagnostic tools of HCC[17], as well as the increased survival rate among patients with cirrhosis to allow time for some of them to develop HCC.
HCC is more prevalent in men than in women[18], which may be at least in part explained by differences in exposure to risk factors. However, sex hormones and other x-linked genetic factors may also be important[19]. It has been speculated that estrogens and androgens could modulate hepatocarcinogenesis and explain the higher incidence of HCC in men[20]. A significant increase in the proportion of HCC among males from 82.5% in periods I to 87.6% in period II (P = 0.009) was observed. The calculated risk of development of HCC was nearly three times higher in men than in women. This is consistent with the findings of Kasahara et al[21], where men were at 4.35 times risk to develop HCC than women.
Analysis of age distribution among HCC patients revealed that the most predominant age group (40-59 years) showed a slight increase in period II compared to period I at the expense of older age group (≥60 years) but the difference did not reach a statistical significance (P = 0.387). This shift to younger age group over the last two decades may be attributed to emergence of HCV infection[22,23], as well as to acquisition of both hepatitis B and C virus infection at younger age[2,24].
Velazquez et al[2], found that cirrhotic patients older than 54 years are at four times greater risk to develop HCC. This is consistent with our findings, where patients of the age group 40-59 years were at 3.7 times and of age group ≥60 years were at 11 times more risk to develop HCC.
Analysis of the risk factors of HCC in patients with CLD has been performed in several studies[25,26]. HBV and HCV infection are considered as the major risk factors that contribute to the development of HCC. During the entire study period, the proportions of HCV-Ab and HBsAg among HCC cases were 86.9% and 28.4% respectively. Surprisingly, there was a significant decline of HBsAg from 38.6% to 20.5% (P = 0.000) and slight increase in HCV-Ab from 85.6% to 87.9% (P = 0.233) in periods I and II respectively. Consequently, the proportion of co-infection of hepatitis C and B declined from 27% to 10.8%. The decline of HBsAg may be partially attributed to successful control measures of blood transfusion introduced in the mid-seventies and partially to the development of mutant or occult HBV infection, which requires costly assays for diagnosis. However, this decline could not be attributed to vaccination programs in children[27], which has been launched in Egypt about 10 years ago. It has been reported that the reduction of the incidence of HBV will be seen 30-40 years after the launch of the universal hepatitis B vaccination program[27]. Although HBV and HCV are both important causes of HCC, data do not suggest, neither do they conclusively refute, a super-additive interaction between the two infections in the development of HCC[28,29].
HBV conferred a higher risk to develop HCC more than HCV (OR 2.5 vs 2.0 respectively). Yet, the low prevalence of HBV render the relative contribution of HBV to HCC (PAR% = 23.4%) lower than HCV contribution (PAR% = 42%). Velazquez and his colleagues[2] in Spain, considered HBV as an insignificant risk factor for development of HCC due to the considerable small number of infected patients as well as low chronicity rate in acute HBV infection (10%) compared to high chronicity rate (50-80%) in acute HCV patients. Studies from earlier years indicated that the rise in the incidence of HCC was directly related to the increase in the prevalence of HCV infection[30]. The prevalence of HCV in Egypt is much more increased[31], which needs to intervene with the acquisition of acute HCV infection as a primary prevention measure. Hepatitis C vaccine is not available; therefore, the rising trend of HCC is most likely attributed to HCV infection[32].
The role of HBV infection in pathogenesis of HCC differs from that of HCV infection; HBV-DNA genome integrates in hepatocellular chromosomes[10], while HCV exerts its effect, most probably, through production of cirrhosis with severe liver damage[30]. The major part of the data shows that HCV plays an indirect role in hepatocarcinogenesis, and this may appear as a consequence of long-standing associated cirrhosis through the direct promotion of the chronic liver inflammation[33]. Recently, many reports point to a direct role of HCV in hepatocarcinogenesis, it is now believed that the core component of HCV may directly participate in the hepatocarcinogenesis[7].
In the current study, viral hepatitis markers were negative in 2.8% of HCC patients in period I and 2.4% of them in period II with no significant difference. A higher percent was reported by El-Serag[1], who found that 14.5% of patients with HCC remain without specific risk factors and diagnosed as non-specific cirrhosis. This could be explained by the development of mutant or occult viral infection or exposure to other risk factors such as aflatoxins, alcohol abuse, or heavy smoking.
A unique invisible risk factor for development of HCC in Egypt could be schistosomal infection and its parenteral therapy. Schistosomiasis induces immune suppression, which could result in increased persistence viremia, following acute infection of both hepatitis B and C[34]. Whereas, parenteral anti-schistosomal therapy had played a role in transmission of HBV and HCV through improperly sterilized glass syringes[35]. In spite that patients with history of schistosomiasis were at higher risk to develop HCC but the results did not reach a statistical significance, which could be attributed to the absence of direct causal effect of schistosomiasis in development of HCC[34].
According to the recommendations of a previous study[3], the awareness about HCC has been greatly improved, which subsequently led to better screening and earlier diagnosis of HCC. This is evidenced by increased rate of diagnosis of small lesions from 14.9% to 22.7% and decreased rate of diagnosis of large HCC lesions from 85.1% to 77.3% in periods I and II respectively.
α-Fetoprotein is the most widely used tumor marker, but has poor diagnostic accuracy and ethnic variability[12]. Diagnostic α-fetoprotein level (≥200 ng/mL) was demonstrated only in 15.6% of cases in period I and 28.9% in period II. The increased proportion of patients with diagnostic α-fetoprotein level may be attributed to the increasing adoption of HCC screening program as well as the use of more sensitive assay methods introduced over the recent years.
These results suggest a rising trend of HCC with increasing risk among HCV-infected men of older age groups; such patients should be carefully followed-up and screened for early detection of HCC.
| 1. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 307] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Rahman El-Zayadi A, Abaza H, Shawky S, Mohamed MK, Selim OE, Badran HM. Prevalence and epidemiological features of hepatocellular carcinoma in Egypt-a single center experience. Hepatol Res. 2001;19:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Ohata K, Hamasaki K, Toriyama K, Ishikawa H, Nakao K, Eguchi K. High viral load is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2004;19:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Xiong J, Yao YC, Zi XY, Li JX, Wang XM, Ye XT, Zhao SM, Yan YB, Yu HY, Hu YP. Expression of hepatitis B virus X protein in transgenic mice. World J Gastroenterol. 2003;9:112-116. [PubMed] |
| 6. | Feitelson M. Hepatitis B virus infection and primary hepatocellular carcinoma. Clin Microbiol Rev. 1992;5:275-301. [PubMed] |
| 7. | Szabó E, Páska C, Kaposi Novák P, Schaff Z, Kiss A. Similarities and differences in hepatitis B and C virus induced hepatocarcinogenesis. Pathol Oncol Res. 2004;10:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Heintges T, Wands JR. Hepatitis C virus: epidemiology and transmission. Hepatology. 1997;26:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Liang TJ, Jeffers LJ, Reddy KR, De Medina M, Parker IT, Cheinquer H, Idrovo V, Rabassa A, Schiff ER. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology. 1993;18:1326-1333. [PubMed] |
| 10. | El-Nady GM, Ling R, Harrison TJ. Gene expression in HCV-associated hepatocellular carcinoma--upregulation of a gene encoding a protein related to the ubiquitin-conjugating enzyme. Liver Int. 2003;23:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Fattovich G. Progression of hepatitis B and C to hepatocellular carcinoma in Western countries. Hepatogastroenterology. 1998;45 Suppl 3:1206-1213. [PubMed] |
| 12. | Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Hennekens CH, Buring JE. Epidemiology in Medicine. Little, Brown and Company, Boston/Toronto. . |
| 14. | Okano H, Shiraki K, Inoue H, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Murata K, Nakano T, Yamakado K. Treatment of hepatocellular carcinoma and the exacerbation of liver function. Int J Oncol. 2001;19:1279-1282. [PubMed] |
| 15. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 16. | Yu MC, Yuan JM, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14:703-709. [PubMed] |
| 17. | El-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87-107, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, Lee SD, Lin CL, Chen PJ, Lin SC. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393-1400. [PubMed] |
| 19. | Yu MC, Tong MJ, Govindarajan S, Henderson BE. Nonviral risk factors for hepatocellular carcinoma in a low-risk population, the non-Asians of Los Angeles County, California. J Natl Cancer Inst. 1991;83:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Nagasue N, Ogawa Y, Yukaya H, Ohta N, Ito A. Serum levels of estrogens and testosterone in cirrhotic men with and without hepatocellular carcinoma. Gastroenterology. 1985;88:768-772. [PubMed] |
| 21. | Kasahara A, Hayashi N, Mochizuki K, Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A, Kiyosawa K, Okuda M. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 334] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Montalto G, Cervello M, Giannitrapani L, Dantona F, Terranova A, Castagnetta LA. Epidemiology, risk factors, and natural history of hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 23. | Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266-269. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 2135] [Article Influence: 79.1] [Reference Citation Analysis (1)] |
| 25. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 537] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Ganne-Carrié N, Chastang C, Chapel F, Munz C, Pateron D, Sibony M, Dény P, Trinchet JC, Callard P, Guettier C. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology. 1996;23:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 27. | Chang MH. Decreasing incidence of hepatocellular carcinoma among children following universal hepatitis B immunization. Liver Int. 2003;23:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Kuper HE, Tzonou A, Kaklamani E, Hadziyannis S, Tasopoulos N, Lagiou P, Trichopoulos D, Stuver S. Hepatitis B and C viruses in the etiology of hepatocellular carcinoma; a study in Greece using third-generation assays. Cancer Causes Control. 2000;11:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 30. | Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 672] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 31. | Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 196] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 32. | Teratani T, Ishikawa T, Shiratori Y, Shiina S, Yoshida H, Imamura M, Obi S, Sato S, Hamamura K, Omata M. Hepatocellular carcinoma in elderly patients: beneficial therapeutic efficacy using percutaneous ethanol injection therapy. Cancer. 2002;95:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Colombo M. Hepatitis C virus and hepatocellular carcinoma. Semin Liver Dis. 1999;19:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Ghaffar YA, Fattah SA, Kamel M, Badr RM, Mahomed FF, Strickland GT. The impact of endemic schistosomiasis on acute viral hepatitis. Am J Trop Med Hyg. 1991;45:743-750. [PubMed] |
| 35. | Madwar MA, el Tahawy M, Strickland GT. The relationship between uncomplicated schistosomiasis and hepatitis B infection. Trans R Soc Trop Med Hyg. 1989;83:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Science Editor Li WZ Language Editor Elsevier HK