Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.4992
Revised: June 8, 2005
Accepted: June 11, 2005
Published online: August 28, 2005
AIM: To observe the biologic behavior of pancreatic cancer cells in vitro and in vivo, and to explore the potential value of angiostatin gene therapy for pancreatic cancer.
METHODS: The recombinant vector pcDNA3.1(+)-angiostatin was transfected into human pancreatic cancer cells PC-3 with Lipofectamine 2000, and paralleled with the vector and mock control. Angiostatin transcription and protein expression were determined by immunofluorescence and Western blot. The stable cell line was selected by G418. The supernatant was collected to treat endothelial cells. Cell proliferation and growth in vitro were observed under microscope. Cell growth curves were plotted. The troms-fected or untroms-fected cells overexpressing angiostatin vector were implanted subcutaneously into nude mice. The size of tumors was measured, and microvessel density count (MVD) in tumor tissues was assessed by immunohistochemistry with primary anti-CD34 antibody.
RESULTS: After transfected into PC-3 with Lipofectamine 2000 and selected by G418, macroscopic resistant cell clones were formed in the experimental group transfected with pcDNA 3.1(+)-angiostatin and vector control. But untreated cells died in the mock control. Angiostatin protein expression was detected in the experimental group by immunofluorescence and Western-blot. Cell proliferation and growth in vitro in the three groups were observed respectively under microscope. After treatment with supernatant, significant differences were observed in endothelial cell (ECV-304) growth in vitro. The cell proliferation and growth were inhibited. In nude mice model, markedly inhibited tumorigenesis and slowed tumor expansion were observed in the experimental group as compared to controls, which was parallel to the decreased microvessel density in and around tumor tissue.
CONCLUSION: Angiostatin does not directly inhibit human pancreatic cancer cell proliferation and growth in vitro, but it inhibits endothelial cell growth in vitro. It exerts the anti-tumor functions through antiangiogenesis in a paracrine way in vivo.
- Citation: Yang DZ, He J, Zhang JC, Wang ZR. Angiostatin inhibits pancreatic cancer cell proliferation and growth in nude mice. World J Gastroenterol 2005; 11(32): 4992-4996
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/4992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.4992
Angiogenesis means the formation of new blood vessels and is indispensable to various physiological processes including reproduction, development, wound repair, and tissue regeneration. There is evidence that tumor growth and metastasis are accompanied with the growth of new blood vessels, which proves the rationality and feasibility of anti-angiogenic therapy for tumor[1,2].
Pancreatic cancer is the second most common cause of death from any type of gastrointestinal diseases in the United States[3]. The common treatment of pancreatic cancer is suboptimal and the prognosis of patients is poor[4].
Although pancreatic cancer is not a grossly vascular tumor, this malignancy often exhibits enhanced foci of endothelial cell proliferation. But several[5-7] studies have reported a positive correlation between blood vessel density. Angiogenesis may play an important role in this disease. Angiostatin plays a key role in regulating endothelial cell proliferation and migration during the process of angiogenesis[8,9] and inhibits the growth of a variety of murine and human tumors[10].
We transfected eukaryotic vector encoding mouse angiostatin into human pancreatic cancer cells to observe the anti-tumor function of angiostatin and to explore the potential activity in gene therapy for pancreatic cancer.
Recombinant vector pcDNA3.1(+)-angiostatin was identified by restriction endonuclease digestion and sequencing. The human pancreatic cancer cell line PC-3 and endothelial cell line ECV-304 were purchased from China Center for Type Culture Collection. PvuI was purchased from Bioson Corporation. Lipofectamine™ 2000 and G418 were purchased from Gibco Company; Rabbit anti-HAtag multiclonl antibody was presented by JichengZhang doctor; FITC was purchased from Boster Biological Technology Co. CD-34 was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co.
Pancreatic cancer cells and endothelial cells were cultured in RPMI1640 (Gibco) supplemented with 10% fetal bovine serum (FBS) (Sijiqing, Hangzhou, China), penicillin (100 U/mL) and streptomycin (100 mg/mL) in a humidified atmosphere of 50 mL/L CO2 at 37 °C.
Pancreatic cancer cells (PC-3) in logarithmic growth phase were implanted in 24-well plates at 2×105 cells/well, and approximately 80% confluence was obtained, after overnight incubation. pcDNA3.1(+)-angiostatin was digested by Pvu I according to the protocol of the manufacturer, and the recombinant was linearized. DNA/Lipofectamine 2000 complexes were prepared and transfected according to the protocol of the manufacturer. The experimental group and the vector control was designed with pcDNA3. 1(+) and the mock control with Lipofectamine 2000. After 12 h of transfection, the selective medium containing G418 (50 mg/L) was used to culture cells for 14 d. Then the isolated resistant cell clones were selected and amplified.
PC-3 with pcDNA3. 1(+) angiostatin in logarithmic growth phase was implanted in 6-well plates. After 24 h, the glass flake was taken out and immunofluorescence cytochemistry was carried out according to manufacturer’s instructions, and the expression was detected under fluorescent microscope.
The cells were cultured in a conditioned media for 5 d. Cell supernatant was mixed with lysine-sepharose and incubated at 4 °C overnight. Detection was performed by the enhanced chemiluminescence (ECL) Western blotting analysis system according to the manufacturer’s instructions.
In logarithmic growth phase, the supernatant was collected. Endothelial cells ECV-304 were treated with supernatant separately for 7 d (supernatant/medium = 1/9), and counted under microscope.
Pancreatic cancer cells (PC-3) and endothelial cells (ECV-304) were plated on 24-well plates at 1×104 cells/well and cultured for 7 d. Viable cells was counted under microscope and growth curves were plotted.
Balbc nude mice were randomly divided into three groups, 5 in each group. The experimental group with pcDNA3.1(+)-angio/PC-3, the vector control with pcDNA3.1(+)/PC-3 and the mock control with untransfected PC-3 were separately injected subcutaneously with pancreatic cancer cells PC-3 at 1.0×106cells each mouse. After 56 d, the mice were killed to measure the size of tumor formed and to calculate the percentage of inhibition on tumorigenesis in vivo.
Tumor tissues were fixed and embedded in paraffin. MVD was detected by immunohistochemistry SP method, and counted in four fields under light microscope (200× selected randomly).
To determine statistical significance, data were analyzed by statistical software of SPSS 10.0, Student’s t test and χ2 test.
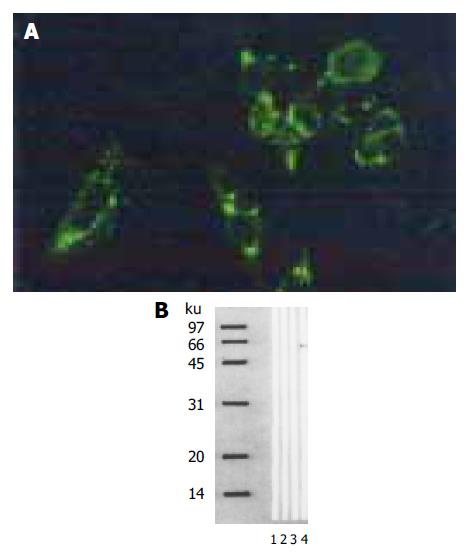
Pancreatic cancer cells (PC-3) transfected with the corresponding vectors were selected by G418 for 14 d. The experimental and vector control groups formed macroscopic cell clones, but the mock group of cells was dead completely after 8 d of selection. Immunofluorescence showed signals of angiostatin in the experimental group of cell clones genetically engineered, but not in the controls (Figure 1A). Western blot analysis of cell supernatants (Figure 1B) revealed the detected protein with the size (5 800 kb) being consistent with angiostatin in the experimental group, whereas no specific band was observed in the controls.
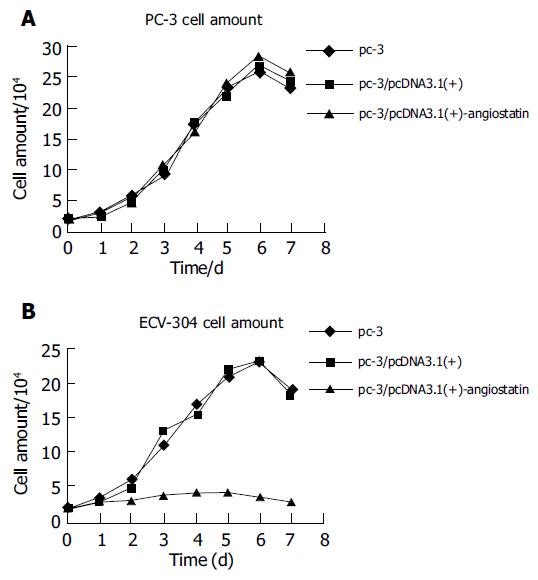
To detect biological activity of the encoded angiostatin in vitro, tumor cells transduced with and without the corresponding vectors were cultured for 7 d to make cell growth curve (Figure 2A). Under microscope, no obvious difference was observed in the cell morphology among the three groups of cells. Cell growth curves indicated no change in cell growth speed and doubling time among the three groups. These results indicated that upregulated angiostatin expression could neither directly inhibit cell growth and proliferation, nor affect cell cycle in vitro. But biological activity of the cell treated with supernatant of PC-3/pcDNA3.1(+)-angiostatin, the growth was obviously inhibited. The growth of the vector and mock control groups was similar to that of normal (Figure 2B). These results indicated that angiostatin expression could directly inhibit endothelial cell growth and proliferation in vitro.
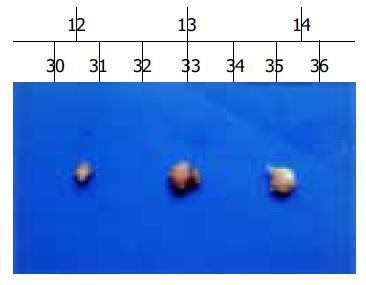
Macroscopic tumors were observed on d 10, after injection of angiostatin developed fast in the vector and mock control groups of nude mice. However, no macroscopic tumors were observed until 14 d, after injection of angiostatin in the experimental group and tumors developed slowly. After 56 d of injection of angiostatin, no mice died in the three groups and the tumors were resected and measured. Small pale tumor nodules were observed in the angiostatin-transfected tumors, whereas red and hypervascularized large tumors were present in the vector-transfected control and mock control tumor cells. The average size of tumors in the experimental group was 6.2×10-2 cm3, much less than the average size of the vector control 1.4×10-2 cm3 and the mock control (Figure 3). The inhibition of angiostatin overexpression reached 77%.
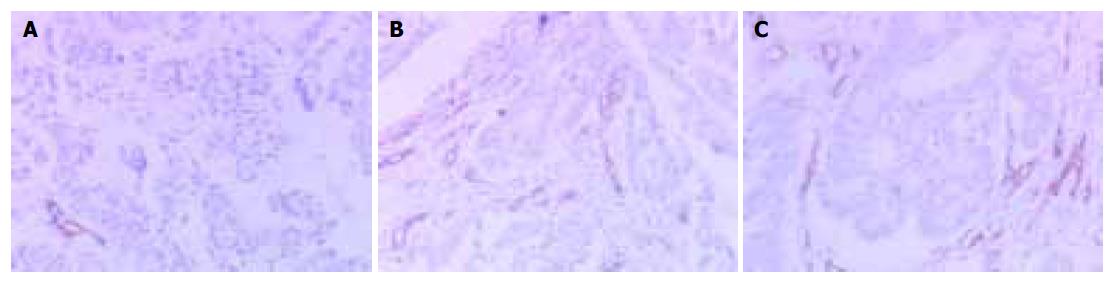
Tumor tissue in the experimental group was more vascularized than in the control groups. Greater microvascular density were found by immunohistochemical staining in tumors in the experimental group than in the vector or mock control groups respectively (P<0.01, Figure 4). The results indicated that overexpression of angiostatin could decrease tumorigenesis by inhibiting neovascularization in tumors in vivo. Angiostatin might have inhibitory actions on surrounding tumor tissues and indirectly inhibit tumorigenesis.
Angiogenesis is critical for normal and pathologic processes in new blood vessel formation and plays an important role in the growth and spread of cancer. New blood vessels “feed” cancer cells with oxygen and nutrients, allow these cells to grow, invade nearby tissue, spread to other parts of the body, and form new colonies of cancer cells. At the prevascular stage, tumor is unable to grow to a size beyond 2-3 mm3 and remains in its dormant state. However, once the angiogenic phenotype of the tumor is switched on, tumor growth rate changes from linear to exponential[11-14].
Angiogenesis begins, when a fibrin clot forms on the adventitial surface of an existing blood vessel[15], followed by sprouting of new capillaries. The initial phase begins with increased vascular permeability and local degradation of the vessel wall. Endothelial cells enter the tumor stroma and proliferate. At this time, the cells may be most vulnerable to agents that interfere with their proliferation, since they lack protection from other cell types[16]. The next step in vessel formation is recruitment of pericytes, followed by smooth muscle cells.
Angiogenesis is a process controlled by certain chemicals produced in the body. These chemicals stimulate cells to repair damaged blood vessels or form new ones such as vascular endothelial growth factor (VEGF)[17], basic fibroblast growth factor (bFGF)[18], acidic fibroblast growth factor (aFGF), Interleukin-8, angiogenin, placental growth factor (PGF)[19], transforming growth factor-TGFα, β[20], other chemicals are inhibitors such as angiostatin[21], thrombospondin-1[22], 16-kd prolactin fragment[23], Interferon-α, β[24], Endostatin[25]. Because cancer cannot grow or spread without the formation of new blood vessels[26], scientists are trying to find ways to stop angiogenesis.
Tumor angiogenesis is often the consequence of an angiogenic imbalance in which pro-angiogenic factors predominate over anti-angiogenic factors[27]. Furthermore, angiogenesis is essential for growth and metastasis of most solid cancers. Pancreatic cancer is not a grossly vascular tumor, but is related to angiogenesis[28]. Anti-angiogenic treatment may be necessary and has potential for treatment of pancreatic cancer.
Angiostatin was first isolated as a circulating angiogenesis inhibitor. The structure of angiostatin includes the first four-kringle domains of plasminogen. Amino acid sequence analysis of kringle domains of human angiostatin has shown that K1, K2, K3, and K4 display considerable similarity (about 50% identity). Among these individual kringles, K1 has been identified as the most potent inhibitor of endothelial cell growth. K3 has higher inhibitory potency than K2. Surprisingly, K4 is virtually inactive in suppression of endothelial cell growth. Indeed, a short version of angiostatin only containing the first three kringle domains without K4 (K1-3) seems to be more active than K1-4 in inhibition of endothelial cell growth[29]. Angiostatin inhibits proliferation of endothelial lineages in a dose-dependant manner, but not in the proliferation of normal and neoplastic nonendothelial cell lines[30]. Human angiostatin inhibits the growth of transplanted human breast carcinoma, colon carcinoma and prostate carcinoma in mice, without obvious weight loss or other toxicity observed. It causes human primary carcinomas to regress to a dormant state by a net balance of tumor cell proliferation and apoptosis[31]. Although angiostatin is a potent inhibitor of angiogenesis and tumor growth, the need of high dosages, repeated injections and long-term administration of this protein has made it less attractive for clinical trials.
In our study, mouse angiostatin cDNA was transfected into human pancreatic cancer cells PC-3, and stable cell lines expressing the secreted angiostatin were proved by immunofluorescence and Western-blot. Although angiostatin has no direct effect on pancreatic cancer cell PC-3 growth in vitro, the supernatant of stable cells inhibited the growth of endothelial cells ECV-304. The stable cell clones were implanted in the back of nude mice and the tumor growth inhibition rate of primary tumor growth was 77%, tumor growth inhibition is related to vascularization. According to the observation, angiostatin exerted its anti-tumor effect through anti-angiogenesis. These results are consistent with previous reports[31]. By transfecting angiostatin into pancreatic cancer cells PC-3, we have gotten the protein. Thus angiostatin gene therapy is for pancreatic cancer. However, long-term, high-level, and sustained expression of angiostatin, are necessary to prevent dormant tumors from becoming active again. To achieve this objective, Xu et al[32] engineered a recombinant adeno-associated virus (AAV) vector encoding mouse angiostatin, and found that it suppresses metastatic liver cancer in mice. In our study, we used Lipofectamine-2000 for transfection and got the similar result. These results support that angiostatin-gene therapy is a potential strategy in the treatment of pancreatic cancer.
In conclusion, angiostatin inhibits pancreatic cancer cell proliferation and growth, and may provide a new way for its treatment.
| 2. | Gasparini G, Longo R, Fanelli M, Teicher BA. Combination of antiangiogenic therapy with other anticancer therapies: results, challenges, and open questions. J Clin Oncol. 2005;23:1295-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Lowenfels AB, Sullivan T, Fiorianti J, Maisonneuve P. The epidemiology and impact of pancreatic diseases in the United States. Curr Gastroenterol Rep. 2005;7:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Horvát-Karajz K. Modern diagnosis and treatment of pancreatic cancer--from the viewpoint of the internist. Orv Hetil. 2005;146:305-308. [PubMed] |
| 5. | Ohshima T, Yamaguchi T, Ishihara T, Yoshikawa M, Kobayashi A, Sakaue N, Baba T, Yamada S, Saisho H. Evaluation of blood flow in pancreatic ductal carcinoma using contrast-enhanced, wide-band Doppler ultrasonography: correlation with tumor characteristics and vascular endothelial growth factor. Pancreas. 2004;28:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Peeters CF, de Geus LF, Westphal JR, de Waal RM, Ruiter DJ, Wobbes T, Oyen WJ, Ruers TJ. Decrease in circulating anti-angiogenic factors (angiostatin and endostatin) after surgical removal of primary colorectal carcinoma coincides with increased metabolic activity of liver metastases. Surgery. 2005;137:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Cao Y. Antiangiogenic cancer therapy. Semin Cancer Biol. 2004;14:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947-C970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 533] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 11. | Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, Reinmuth N. Overview of angiogenesis: Biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol. 2000;50:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Giuliani N, Colla S, Rizzoli V. Angiogenic switch in multiple myeloma. Hematology. 2004;9:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Egginton S, Zhou AL, Brown MD, Hudlická O. The role of pericytes in controlling angiogenesis in vivo. Adv Exp Med Biol. 2000;476:81-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Sun FY, Guo X. Molecular and cellular mechanisms of neuroprotection by vascular endothelial growth factor. J Neurosci Res. 2005;79:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Sauer G, Deissler H. Angiogenesis: prognostic and therapeutic implications in gynecologic and breast malignancies. Curr Opin Obstet Gynecol. 2003;15:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Dalal S, Berry AM, Cullinane CJ, Mangham DC, Grimer R, Lewis IJ, Johnston C, Laurence V, Burchill SA. Vascular endothelial growth factor: a therapeutic target for tumors of the Ewing's sarcoma family. Clin Cancer Res. 2005;11:2364-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Folkman J. Looking for a good endothelial address. Cancer Cell. 2002;1:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Canfield AE, Schor AM, Schor SL, Grant ME. The biosynthesis of extracellular-matrix components by bovine retinal endothelial cells displaying distinctive morphological phenotypes. Biochem J. 1986;235:375-383. [PubMed] |
| 21. | Narizhneva NV, Razorenova OV, Podrez EA, Chen J, Chandrasekharan UM, DiCorleto PE, Plow EF, Topol EJ, Byzova TV. Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005;19:1158-1160. [PubMed] |
| 22. | Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, Takakura N. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105:2757-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Izawa JI, Sweeney P, Perrotte P, Kedar D, Dong Z, Slaton JW, Karashima T, Inoue K, Benedict WF, Dinney CP. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res. 2002;8:1258-1270. [PubMed] |
| 24. | Folkman J. Endogenous angiogenesis inhibitors. APMIS. 2004;112:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6572] [Article Influence: 252.8] [Reference Citation Analysis (0)] |
| 26. | Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647-654. [PubMed] |
| 27. | Onizuka S, Kawakami S, Taniguchi K, Fujioka H, Miyashita K. Pancreatic carcinogenesis: apoptosis and angiogenesis. Pancreas. 2004;28:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Geiger JH, Cnudde SE. What the structure of angiostatin may tell us about its mechanism of action. J Thromb Haemost. 2004;2:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | van Moorselaar RJ, Voest EE. Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. 2002;197:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Fukumori T, Nishitani Ma, Naroda T, Onishi T, Oka N, Kanayama Ho, Kagawa S. Expression of angiostatin cDNA in a murine renal cell carcinoma suppresses tumor growth in vivo. Urology. 2002;59:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Jung SP, Siegrist B, Wang YZ, Wade MR, Anthony CT, Hornick C, Woltering EA. Effect of human Angiostatin protein on human angiogenesis in vitro. Angiogenesis. 2003;6:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Xu R, Sun X, Tse LY, Li H, Chan PC, Xu S, Xiao W, Kung HF, Krissansen GW, Fan ST. Long-term expression of angiostatin suppresses metastatic liver cancer in mice. Hepatology. 2003;37:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK