Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.4986
Revised: January 8, 2005
Accepted: January 12, 2005
Published online: August 28, 2005
AIM: To study the protective effect of Astragalus membranaceus on intestinal mucosa reperfusion injury and its mechanism after hemorrhagic shock in rats.
METHODS: A total of 32 SD rats were randomly divided into four groups (n = 8, each group): normal group, model group, low dosage group (treated with 10 g/kg Astragalus membranaceus) and high dosage group (treated with 20 g/kg Astragalus membranaceus). The model of hemorrhagic shock for 60 min and reperfusion for 90 min was established. Therapeutic solution (3 mL) was administrated before reperfusion. At the end of the study, the observed intestinal pathology was analyzed. The blood concentrations of lactic acid (LD), nitric oxide (NO), endothelin-1 (ET-1), malondialdehyde (MDA) and the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) in intestinal mucosa were determined.
RESULTS: The intestinal mucosa pathology showed severe damage in model group and low dosage group, slight damage in high dosage group and no obvious damage in normal group. The Chiu’s score in low dose group and high dose group was significantly lower than that in model group. The content of MDA in model group was higher than that in low and high dose groups, while that in high dose group was almost the same as in normal group. The activity of SOD and GSH-PX was the lowest in model group and significantly higher in high dose group than in normal and low dose groups. The concentrations of LD and ET-1 in model group were the highest. The concentrations of NO in model group and low dose group were significantly lower than those in high dose group and normal group.
CONCLUSION: High dose Astragalus membranaeus has much better protective effect on hemorrhagic shock-reperfusion injury of intestinal mucosa than low dose Astragalus membranaceus. The mechanism may be that Astragalus membranaceus can improve antioxidative effect and regulate NO/ET level during hemorrhagic reperfusion.
-
Citation: Hei ZQ, Huang HQ, Zhang JJ, Chen BX, Li XY. Protective effect of
Astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J Gastroenterol 2005; 11(32): 4986-4991 - URL: https://www.wjgnet.com/1007-9327/full/v11/i32/4986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.4986
Under normal conditions , the integrity of intestinal mucosa as a barrier, can prevent bacterial translocation[1,2]. Bacterial and endotoxin can enter into blood across the barrier when the intestinal mucosal barrier is demolished due to anoxia, ischemic and reperfusion injury. It is possible to induce systemic inflammatory response syndrome (SIRS) or multiple organ dysfunction syndrome (MODS), leading to the hemorrhagic shock[3-6].
Free oxygen radical is one of the major activating factors in ischemia/reperfusion injury of intestinal mucosa[7]. The maladjustment of NO/ET can not only aggravate oxidative damage, but also lead to dysfunction of the microcirculation of intestinal mucosa[8-11]. Many drugs have been tried to palliate the ischemia-reperfusion injury of intestinal mucosa after hemorrhagic shock[8,12,13]. However the results are still controversial and unsatisfactory.
Astragalus membranaceus is a traditional Chinese medicine which can improve microcirculation and has good curative effect. The main purpose of our study was to investigate whether Astragalus membranaceus could protect intestinal mucosa against ischemia-reperfusion injury after hemorrhagic shock, and to observe the effect of Astragalus membranaceus on intestinal oxidative damage, NO and ET levels.
Approved by the University Animal Study Committee, 32 healthy male Sprague-Dawley rats (200-300 g, provided by Animal Center of Sun Yat-Sen University) were randomly divided into four groups: normal group (n = 8, sham-operation, no shock and reperfusion), model group (n = 8, with hemorrhagic shock and treated only with 3 mL normal saline intravenously prior to reperfusion), low dose group (n = 8, with hemorrhagic shock and treated with 10 mg/kg Astragalus membranaceus which was five times of the human clinical dose) and high dose group (n = 8, with hemorrhagic shock and treated with 20 mg/kg of Astragalus membranaceus which was 10 times of the human clinical dose). The latter three groups were experimental groups.
Astragalus membranaceus (provided by Dioujiuhong Pharmaceutical Co., Ltd, Chengdu, China) was diluted in 3 mL normal saline and infused intravenously for 3 min prior to reperfusion.
Laboratory temperature was kept at 25-27 °C. The rats were anesthetized by intraperitoneal injection of urethane (5.0 mL, 20%) after they were fasted for 24 h. Tracheotomy was performed for ventilation. The right cervical vein was cannulated for monitoring central venous pressure and fluid infusion and drugs. The left carotid artery and femoral artery were catheterized for monitoring arterial pressure. The femoral artery was used to withdraw blood samples and to create hemorrhagic shock model.
Hemorrhagic shock model was established by withdrawing blood by femoral artery until mean arterial blood pressure (MABP) reached about 5.3 kPa (40 mmHg) and maintained for 60 min.
Rats were administrated with 3 mL solution/drugs. Then their blood was reinfused for approximately 5 min and observed for 90 min. The segment samples of small intestinal mucosa were taken. The rats were killed at the end of the experiment.
The parameters included room temperature, rat weight and blood loss.
Mean arterial pressure and pulse rate were recorded every 10 min during the hemorrhagic shock and resuscitation period.
After successful establishment of experimental model, the rats were killed and paunched rapidly. A segment of 0.5-1.0 cm intestine was cut from 5 cm to terminal ileum, fixed in 4% formaldehyde polymerisatum and embedded in paraffin for section. Another segment of small intestine was washed with frozen saline. The intestinal mucosa was scraped off, dried with suction paper and preserved at -70 °C.
The segment of small intestine was stained with hematoxylin-eosin. The damages of intestinal mucosa were evaluated by criteria of modified Chius method. Criteria of modified Chiu grading system were divided into 10 subdivisions according to the changes of villus and gland of intestinal mucosa: 0, normal villus and gland; 1, changes in top of villus and initial formation of subepidermal Gruenhagen’s antrum; 2, formation of subepidermal Gruenhagen’s antrum and slightly injured gland; 3, enlargement of subepidermal gap and engorgement of capillary vessel; 4, epidermis moderately isolated with lamina propria and injured gland; 5, top villus shedding; 6, obvious villus shedding and capillary vessel dilating; 7, lamina propria villus shedding ,and distinct injured gland; 8, initially decomposed lamina propria; 9, hemorrhage and ulcer.
Intestinal mucosal tissues were weighed and made into 10% homogenate. The lactic acid content in tissues was determined by the method of minim quick measuration (Jiancheng Bioengineering Ltd, Nanjing, China) and the concentration of protein was determined by Coomassie brilliant blue. The results were expressed as mmol/gpro.
Intestinal mucosal tissues (100 mg) were homogenized with normal saline. MDA content was determined by TBA method (Jiancheng Bioengineering Ltd, Nanjing, China). Homogenate (0.1 mL) was taken to detect MDA content. Briefly, 0.1 mL 8.1% SDS, 0.8 mL acetic acid buffer, 0.8 mL 0.8% TBA and 0.2 mL distilled water were added into the sample tubes and one standard tube (containing 0.1 mL tetraethoxypropane). Then all the tubes were incubated at 100 °C for 1 h. After cooled at -20 °C for 5 min, 2 mL n-butyl alcohol was added into the sample, which was then vibrated for 1 min and centrifuged for 10 min at 3 000 r/min. The supernatant of the samples was taken to detect absorbance at 533 nm with spectrophotometer(content of MDA (nmol/100 mg) = absorbance of each sample/absorbance of standard×dilution times).
Intestinal mucosal tissues (100 mg) were weighed and made into 10% homogenate with 0.9 mL normal saline , frozen in refrigerator at -20 °C for 5 min and centrifuged for 15 min at 4 000 r/min. Supernatants were transferred into fresh tubes for the evaluation of SOD activity. SOD activity was evaluated with SOD detection kit according to the manufacturer’s instructions(Kits were provided by Jiancheng Bioengineering Ltd , Nanjing, China). SOD activity (μ/mL) = (A0-A1)/A0×360×V (A0: absorbance of self-oxidation rate per minute; A1: absorbance of each sample per minute; V: volume of extracted tissues).
Intestinal mucosal tissues (100 mg) were weighed and made into 10% homogenate with 0.9 mL normal saline. The activity of GSH-PX was detected according to the manufacturer’s instructions (reagents were purchased from Jiancheng Bioengineering Ltd, Nanjing, China). Protein of homogenates was detected with Coomassie brilliant blue. The calculated results were expressed by U/100 mg protein.
Intestinal mucosal tissues (100 mg) were weighed and made into 10% homogenate with 0.9 mL normal saline. After being centrifuged for 10 min at 10 000 g, the supernatants were placed in boiling water for 3 min and then centrifuged for 5 min at 10 000 g. Supernatant (0.1 mL) was taken for detection, 0.2 mL 35% sulfosalicylic acid was added into the sample to make protein deposits. The sample was homogenized and centrifuged at 10 000 g for 10 min. The supernatants were taken again and preserved at -20 °C in refrigerator. One hundred μL supernatant was detected by indirect nitric acid deoxidized enzyme method (Kits were provided by Jingmei Bioengineering Ltd). After 100 μL nitrate reductase was homogenized gently, the sample was placed in water at 37 °C for 1 h with KNO2 standard succi homogenized. After being placed in ambient temperature for 10 min and with zero setting with blank tube at 530 nm wavelength and 0.5 cm colorimetric cylinder, A value of detection tube and standard tube was read respectively.
Intestinal mucosal tissues (100 mg) were weighed and made into 10% homogenate with 0.9 mL normal saline. Homogenate ET-1 levels were measured by radioim-munoassay (Kits were obtained from Beijing East Asian Radioimmunoassay Technology Institute, Beijing, China).
Data were expressed as mean±SD and analysis of variance was performed using SPSS10.0 software. One-way analysis of variance was used for multiple comparisons and least significant difference test (LSD-t) was used for intra-group comparison. P<0.05 was considered statistically significant.
There was no difference in the four groups (P>0.05). The amount of withdrawn blood in three groups was similar, except in normal group (Table 1).
| Group | n | Weight (g) | Ambienttemperature (°C) | Bloodlettingvolume (mL) |
| Normal | 8 | 265±18 | 26.38±1.06 | -------- |
| Model | 8 | 281±20 | 26.67±0.52 | 5.10±1.80 |
| Low dose | 8 | 269±14 | 25.88±1.13 | 5.20±1.60 |
| High dose | 8 | 272±16 | 26.20±1.10 | 4.80±1.50 |
There was no difference in MAP and HR in four groups before the experiment (P>0.05). The MAP in experimental groups was maintained at 5.33 kPa (40 mmHg), but the HR decreased during hemorrhage shock period. There was no significant difference after resuscitation in experimental group (P>0.05, Table 2).
| Groups /n | Pre-shock | 30 min of shock | 10 min after reperfusion | 30 min after reperfusion | 60 min after reperfusion | 90 min after reperfusion | |
| Normal | MABP | 12.66±1.28 | |||||
| ( n = 8) | HR | 316.50±22.12 | -------- | ------ | |||
| Model | MABP | 12.68±0.82 | 5.33±0.25 d | 12.66±1.35 | 12.83±1.60 | 11.65±0.68 | 11.60±1.82 |
| (n = 8) | HR | 322.50±28.12 | 239.75±35.50 d | 256.40±35.63b | 292.55±41.96 | 325.36±36.81 | 309.25±38.68 |
| Low dose | MABP | 13.07±1.27 | 5.32±0.20 d | 13.21±1.17 | 12.46±1.46 | 12.21±0.82 | 12.05±1.58 |
| (n = 8) | HR | 320.87±38.83 | 221.75±49.89 d | 250.00±45.63b | 282.76±45.63 | 319.50±42.28 | 300.62±32.28 |
| High dose | MABP | 13.00±1.36 | 5.30±0.20 d | 12.16±1.35 | 12.90±1.60 | 12.05±0.82 | 11.82±1.42 |
| (n = 8) | HR | 310.83±25.63 | 236.16±51.67b | 266.00±38.10 b | 316.83±30.91 | 306.66±22.11 | 298.50±22.11 |
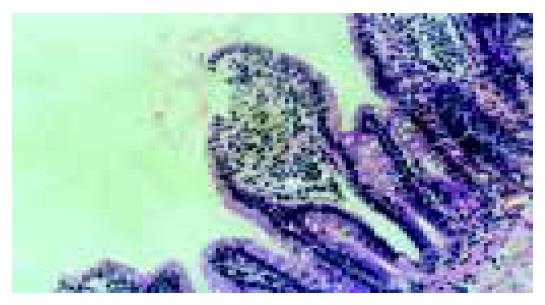
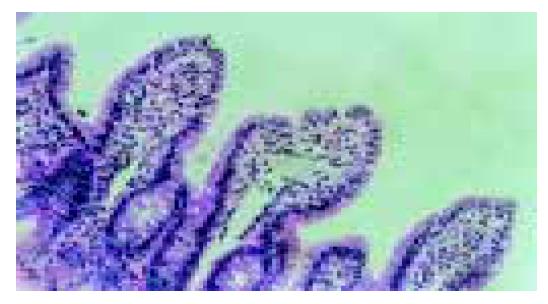
The villus and glands were normal and no inflammatory cell infiltration was observed in mucosal epithelial layer in normal group. Severe edema of mucosa villus and infiltration of necrotic epithelial and inflammatory cells were found, indicating that damage was severe in model group. Light edema of mucosa villus and infiltration of few necrotic epithelial inflammatory cells were found in mucosa epithelial layer in low dosage group. No significant edema and necrotic mucosa villus were observed, but infiltration of a few inflammatory cells in mucosal epithelial layzer was found in high dosage group, which was also the same as those in normal intestinal mucosa (Figures 1, Figures 2, Figures 3, Figures 4).
The Chiu’s score was the lowest in normal group and the highest in model group. Compared to model group, the Chiu’s score was significantly lower in high dose group (P<0.05, Table 3).
| Group | n | Chiu‘s score | Lactic acidnmol/gpro | NOnmol/100 mg | ETPg/100 mg | MDA contentnmol/100 mg | SOD activity(U/100 mg) | GSH-PX activity(U/100 mg) |
| Normal | 8 | 0.90±0.86 | 2.62±0.45 | 41.27±8.60 | 274.62±44.2 | 28.89±4.90 | 42.42±4.37 | 58.04±7.18 |
| Model | 8 | 6.25±2.75b | 3.11±0.53a | 20.21±4.14b | 481.50±109.98b | 62.70±15.37b | 27.85±10.75b | 42.92±10.62a |
| Low dosage | 8 | 4.05±1.96bc | 3.00±0.36a | 26.86±5.33ae | 414.53±26.15bce | 36.80±9.35bd | 39.13±7.62ace | 55.91±11.27ae |
| High dosage | 8 | 3.27±1.82ac | 2.69±0.19a | 42.41±9.89d | 353.51±31.90ad | 31.31±11.45d | 54.38±6.84ad | 77.78±13.56bd |
The content of MDA in intestinal mucosa was the highest in model group, compared to that in normal, high and low dose groups. However there was no difference in Chiu’s score among normal, low and high dose groups (P>0.05, Table 3).
The activity of SOD in intestinal mucosa was the highest in high dose group (P<0.05 or 0.01), and the lowest in model group (P<0.05, Table 3).
The activity of GSH-PX was the lowest in model group (P<0.05) compared to that in the other three groups. There was no difference between normal group and low dose group (P>0.05, Table 3).
The content of lactic acid in model group and low dose group was significantly higher than that in normal and high dose groups (P<0.05), There was no significant difference among model group, low and high dose groups (P>0.05, Table 3).
The content of NO in small intestinal mucosa in model group and low dosage group was significantly lower than that in normal group and high dosage group (P<0.05 or 0.01). There was no significant difference between model group and low dosage group (P>0.05) and between normal group and high dosage group (P>0.05, Table 3).
The content of ET-1 in intestinal mucosa was significantly higher in three experimental groups (P<0.01). The content of ET-1 in model group was higher than that in low and high dose groups (P<0.05 or 0.01, Table 3) .
Blood supply of small intestine from celiac branch of superior mesenteric artery accounts for 20% of total body blood volume. The blood flow in mucous layer is about 70-80% of total intestine blood flow, while the blood flow in muscular layer and serosa is about 15-25% of intestine blood flow. The blood flow in submucous layer is less than 5% of intestine blood flow. But 60% of blood flow in mucous layer is concentrated on the top of mucosa which provides enough hemoperfusion of endotheliocytes and villus[14]. When shock occurs, the blood flow in intestinal mucosa decreases sharply. That is why the small intestinal mucosa is most easily subjected to ischemia and reperfusion injury[15,16].
Studies have proved that ischemia reperfusion injury of intestinal mucosa plays an important role in inducing multiple organ disfunction syndrome (MODS)[3-6]. Therefore prevention of intestinal mucosal barrier from hemorrhagic shock is very important. The results of our study showed that small intestinal mucosa was severely injured after simple hemorrhagic shock. However, the injury of small intestinal villus was alleviated after Astragalus membranaceus was administrated. The best result was obtained with high dose Astragalus membranaceus treatment, indicating that Astragalus membranaceus can protect intestinal mucosa against reperfusion injury, after hemorrhagic shock in a dose-dependant manner.
NO and ET-1 are active substances released by vascular endothelial cells ( especially in lung ) and the most important endogenous regulatory factors. It is most important to keep the dynamic balance between NO and ET in order to maintain tissue and organ hemoperfusion. Some studies[9,17,18] reported that NO and ET-1 increase in plasma sharply during hemorrhagic shock and after resuscitation. At the same time, the intestinal barrier is also damaged.
NO/ET imbalance and endothelial disorder can induce intestinal mucosal hypoperfusion or ischemic injury, when hemorrhagic shock occurs.
Our study showed lactic acid content in intestinal mucosa in model group increased markedly, suggesting that anaerobic metabolism of intestinal mucosa occurs. NO level decreased and ET level increased sharply, suggesting that microcirculation of the intestinal mucosa is destroyed during and after hemorrhagic shock /reperfusion injury.
Bauer et al[19,20] found that intestinal damage can be relieved by drugs, when endogenous NO release is evoked. Oktar et al[21] and Anadol et al[22] used ET receptor antagonists to suppress endogenous ET activity, and found that they can relieve the damage due to intestine ischemic reperfusion, demonstrating that ET receptor antagonists can relieve ischemia reperfusion injury of intestine by modulating NO/ET level.
We found that Astragalus membranaceus could increase endogenous NO level and decrease endogenous ET level in intestinal mucosa, suggesting that Astragalus membranaceus can relieve endothelial dysfunction and ameliorate microcirculation via regulating NO/ET level during hemorrhagic-reperfusion.
Oxygen free radical is another major factor in inducing ischemia reperfusion injury. It could damage the structure of cell membrane and mitochondrial membrane through lipid peroxidation and result in cellular structure destroy and cell dysfunction[7,8]. MDA is the direct products of lipid peroxidation[7,8]. The extent of lipid peroxidation could be accessed by measuring MDA level in tissues. Our study also showed that the content of MDA in intestinal mucosa in model group was significantly higher than that in other groups, suggesting that significant lipid peroxidation occurs in small intestinal mucosa during hemorrhagic shock and reperfusion period. Some previous studies found[23,24] that peroxide dismutase significantly relieves small intestine mucosal injury after 3 h ischemia, demonstrating that oxygen free radical plays an important role in ischemic injury of small intestinal mucosa.
SOD and GSH-PX are the major enzymes for scavenging oxygen free radical, whose activity could reflect its functional status[25-27]. The activity of SOD and GSH-PX in Astragalus membranaceus-treated groups was markedly higher than that in normal group and model group, which would be beneficial to scavenging oxygen free radical.
The content of MDA in intestinal mucosa in Astragalus membranaceus-treated groups was obviously lower than that in model group, demonstrating that Astragalus membranaceus has powerful antioxidative effects and protects small intestinal mucosa against hemorrhagic- reperfusion injury.
In conclusion, Astragalus membranaceus protects intestinal mucosa against hemorrhagic-reperfusion injury in a dose-dependant manner by regulating NO/ET level of intestinal mucosa after ischemia-reperfusion.
The authors thank Professor Wei-Kan Wu for his good advice and Hui-Lan Sun for assistance to the exeperiments.
| 1. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 392] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol Cell Physiol. 2001;280:C343-C351. [PubMed] |
| 4. | Guo W, Magnotti LJ, Ding J, Huang Q, Xu D, Deitch EA. Influence of gut microflora on mesenteric lymph cytokine production in rats with hemorrhagic shock. J Trauma. 2002;52:1178-1185; disciussion 1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Pape HC, Grotz M, Remmers D, Dwenger A, Vaske R, Wisner D, Tscherne H. Multiple organ failure (MOF) after severe trauma--a sheep model. Intensive Care Med. 1998;24:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bedirli A, Sözüer EM, Muhtaroğlu S, Alper M. The role of oxygen free radicals and nitric oxide in organ injury following hemorrhagic shock and reinfusion. Int J Surg Investig. 2000;2:275-284. [PubMed] |
| 8. | Mota-Filipe H, McDonald MC, Cuzzocrea S, Thiemermann C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Hierholzer C, Kalff JC, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2004;286:G225-G233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Massberg S, Boros M, Leiderer R, Baranyi L, Okada H, Messmer K. Endothelin (ET)-1 induced mucosal damage in the rat small intestine: role of ET(A) receptors. Shock. 1998;9:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Denizbaşi A, Yegen C, Oztürk M, Yegen B. Role of nitric oxide in gastric injury induced by hemorrhagic shock in rats. Pharmacology. 2000;61:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Izumi M, McDonald MC, Sharpe MA, Chatterjee PK, Thiemermann C. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock. 2002;18:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Wattanasirichaigoon S, Menconi MJ, Fink MP. Lisofylline ameliorates intestinal and hepatic injury induced by hemorrhage and resuscitation in rats. Crit Care Med. 2000;28:1540-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 210] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Morini S, Yacoub W, Rastellini C, Gaudio E, Watkins SC, Cicalese L. Intestinal microvascular patterns during hemorrhagic shock. Dig Dis Sci. 2000;45:710-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. 2001;164:1526-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Fruchterman TN, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417-422. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Mailman D. Modulation of hemorrhagic shock by intestinal mucosal NG-nitro-L-arginine and L-arginine in the anesthetized rat. Shock. 1999;12:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Bauer C, Kuntz W, Ohnsmann F, Gasser H, Weber C, Redl H, Marzi I. The attenuation of hepatic microcirculatory alterations by exogenous substitution of nitric oxide by s-nitroso-human albumin after hemorrhagic shock in the rat. Shock. 2004;21:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kawata K, Takeyoshi I, Iwanami K, Sunose Y, Aiba M, Ohwada S, Matsumoto K, Morishita Y. A spontaneous nitric oxide donor ameliorates small bowel ischemia-reperfusion injury in dogs. Dig Dis Sci. 2001;46:1748-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Oktar BK, Gulpinar MA, Bozkurt A, Ghandour S, Cetinel S, Moini H, Yegen BC, Bilsel S, Granger DN, Kurtel H. Endothelin receptor blockers reduce I/R-induced intestinal mucosal injury: role of blood flow. Am J Physiol Gastrointest Liver Physiol. 2002;282:G647-G655. |
| 22. | Anadol AZ, Bayram O, Dursun A, Ercan S. Role of endogenous endothelin peptides in intestinal ischemia-reperfusion injury in rats. Prostaglandins Leukot Essent Fatty Acids. 1998;59:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Akcakaya A, Alimoglu O, Sahin M, Abbasoglu SD. Ischemia-reperfusion injury following superior mesenteric artery occlusion and strangulation obstruction. J Surg Res. 2002;108:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Riaz AA, Wan MX, Schäfer T, Dawson P, Menger MD, Jeppsson B, Thorlacius H. Allopurinol and superoxide dismutase protect against leucocyte-endothelium interactions in a novel model of colonic ischaemia-reperfusion. Br J Surg. 2002;89:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Cuzzocrea S, Mazzon E, Dugo L, Caputi AP, Aston K, Riley DP, Salvemini D. Protective effects of a new stable, highly active SOD mimetic, M40401 in splanchnic artery occlusion and reperfusion. Br J Pharmacol. 2001;132:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Muzakova V, Kandar R, Vojtisek P, Skalicky J, Cervinkova Z. Selective antioxidant enzymes during ischemia/reperfusion in myocardial infarction. Physiol Res. 2000;49:315-322. |
| 27. | Akçil E, Tuğ T, Döşeyen Z. Antioxidant enzyme activities and trace element concentrations in ischemia-reperfusion. Biol Trace Elem Res. 2000;76:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK