Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4827
Revised: December 3, 2004
Accepted: December 8, 2004
Published online: August 21, 2005
AIM: To investigate the response of astrocytes and neurons in rat lumbo-sacral spinal cord and medulla oblongata induced by chronic colonic inflammation, and the relationship between them.
METHODS: Thirty-three male Sprague-Dawley rats were randomly divided into two groups: experimental group (n = 17), colonic inflammation was induced by intra-luminal administration of trinitrobenzenesulfonic acid (TNBS); control group (n = 16), saline was administered intra-luminally. After 3, 7, 14, and 28 d of administration, the lumbo-sacral spinal cord and medulla oblongata were removed and processed for anti-glial fibrillary acidic protein (GFAP), Fos and GFAP/Fos immunohistochemistry.
RESULTS: Activated astrocytes positive for GFAP were mainly distributed in the superficial laminae (laminae I-II) of dorsal horn, intermediolateral nucleus (laminae V), posterior commissural nucleus (laminae X) and anterolateral nucleus (laminae IX). Fos-IR (Fos-immunoreactive) neurons were mainly distributed in the deeper laminae of the spinal cord (laminae III-IV, V-VI). In the medulla oblongata, both GFAP-IR astrocytes and Fos-IR neurons were mainly distributed in the medullary visceral zone (MVZ). The density of GFAP in the spinal cord of experimental rats was significantly higher after 3, 7, and 14 d of TNBS administration compared with the controls (50.4±16.8, 29.2±6.5, 24.1±5.6, P<0.05). The density of GFAP in MVZ was significantly higher after 3 d of TNBS administration (34.3±2.5, P<0.05). After 28 d of TNBS administration, the density of GFAP in the spinal cord and MVZ decreased and became comparable to that of the controls (18.0±4.9, 14.6±6.4, P>0.05).
CONCLUSION: Astrocytes in spinal cord and medulla oblongata can be activated by colonic inflammation. The activated astrocytes are closely related to Fos-IR neurons. With the recovery of colonic inflammation, the activity of astrocytes in the spinal cord and medulla oblongata is reduced.
- Citation: Sun YN, Luo JY, Rao ZR, Lan L, Duan L. GFAP and Fos immunoreactivity in lumbo-sacral spinal cord and medulla oblongata after chronic colonic inflammation in rats. World J Gastroenterol 2005; 11(31): 4827-4832
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4827.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4827
Recent studies have shown that astrocytes, being in intimate contact with neurons, may respond to various kinds of stimulation. Both peripheral inflammation and nerve injuries are able to induce astrocytic activation in the spinal cord and brain stem. Moreover, astrocytic activation was shown to be involved in central nervous system (CNS) responses leading to hypersensitivity and persistent pain states[1-3]. Glial fibrillary acidic protein (GFAP) is an intermediate filament component in astrocytes of nervous tissue. The expression of GFAP may increase with astrocytic activation[4]. By using GFAP and Fos immunoreactivity (IR) as markers, we aimed to determine the response of astrocytes and neurons in rat lumbo-sacral spinal cord and medulla oblongata induced by chronic colonic inflammation, and the relationship between them.
Thirty-three adult male Sprague-Dawley rats (The Fourth Military Medical University) weighing 220-250 g were used in this study. The animals were housed in a quiet room with a constant ambient temperature of 20 °C, and free access to rat chow and water. They were divided randomly into experimental group (n = 17) and control group (n = 16).
After 24 h fast, trinitrobenzenesulfonic acid (TNBS, 100 mg/kg in 300 mL/L ethanol) was administered intra-luminally through a silicone rubber catheter introduced 7 cm into the anus with light diethyl-ether anesthesia, as previously described[5]. To keep TNBS in the colon for a longer time and to avoid leakage, the tubing was slowly withdrawn and the tail of the rat was kept elevated for 8-10 min. After intra-luminal administration of TNBS, rats in the experimental group were allowed to live 3 (n = 4), 7 (n = 4), 14 (n = 4) and 28 (n = 5) d, respectively.
After intra-luminal administration of 0.5 mL saline, rats in the control group were allowed to live 3 (n = 4), 7 (n = 4), 14 (n = 4) and 28 (n = 4) d, respectively.
The animals were deeply anesthetized with pentobarbital Na (80 mg/kg, intraperitoneal) and perfused intracardially with 100 mL saline followed by 500 mL fixative of 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4). L3-S2 segments of the spinal cord and medulla oblongata were removed post-fixed in the same fixative at 4 °C for 2-4 h and then cryoprotected in 20% sucrose overnight. Serial frozen sections of 40 μm thick were cut on a Leitz cryocut. Sections were collected in 0.01 mol/L PBS for immunohistochemistry.
The spinal cord and medulla oblongata sections from each rat were randomly divided into three sets. Two sets were processed for anti-GFAP and anti-Fos immunohistochemical staining by using avidin-biotin-peroxidase complex (ABC) method. Briefly, free-floating tissue sections were treated in 800 mL/L methanol containing 0.3% H2O2 to block endogenous peroxidase activity for 30 min at room temperature. They were treated with 0.01 mol/L PBS containing 0.1% Triton X100 for 20 min at room temperature. The sections were then incubated with a polyclonal rabbit anti-Fos antibody (1:3 000, Santa Cruz) or rabbit anti-GFAP antibody (1:3 000 Dako) for 48 h at 4 °C. After that, the sections were incubated with biotinylated goat anti-rabbit IgG (1:500, Sigma), and subsequently with the ABC complex (1:500, Sigma) at room temperature for 2 h each. The antigen朼ntibody reaction sites were visualized by incubation with glucose oxidase-DAB-nickel method for 15-30 min at room temperature. The sections were rinsed in 0.01 mol/L PBS for 3 min10 min during the transition of these steps. The other set group of sections was stained for GFAP and Fos by using double immunohistochemical labeled method. Finally, the sections were mounted onto gelatin-coated slides, dried, dehydrated, cleared and coverslipped.
Colon specimens that were 1 cm long and 7 cm proximal to the anus were taken for histological assessment. They were rapidly immersed in cold 100 mL/L neutral buffered formalin, fixed overnight. Then they were processed routinely and embedded in paraffin blocks, and 3-5 μm-thick cross sections were stained with H&E and examined by light microscope.
The density of GFAP staining and the numbers of neuronal nuclear profiles expressing Fos were analyzed with computer assisted QUIC Menu System. The ratio of the area of stained GFAP to the area of outlined regions was presented as the density of GFAP. Fos counting was done by outlining specific regions and then, particles (stained nuclei) were counted in the outlined regions.
For each animal, the average for density measurements and number counts in the spinal cord was obtained unilaterally from five randomly selected sections. In the medulla oblongata, four sections were selected from above the obex, obex, the area postrema, and pyramidal decussation levels respectively. The average for density measurements and number counts was obtained unilaterally in the nucleus of the solitary tract (NTS), ventrolateral medulla (VLM) and intermediate reticular zone (IRt) from those four section levels.
Pictures were taken under BX-60 microscope with the support of IM50 software. All results are expressed as mean±SD. An unpaired t-test was used to compare GFAP density and Fos-IR cell count data between the groups. A difference was accepted as significant, if the probability was less than 5% (P<0.05).
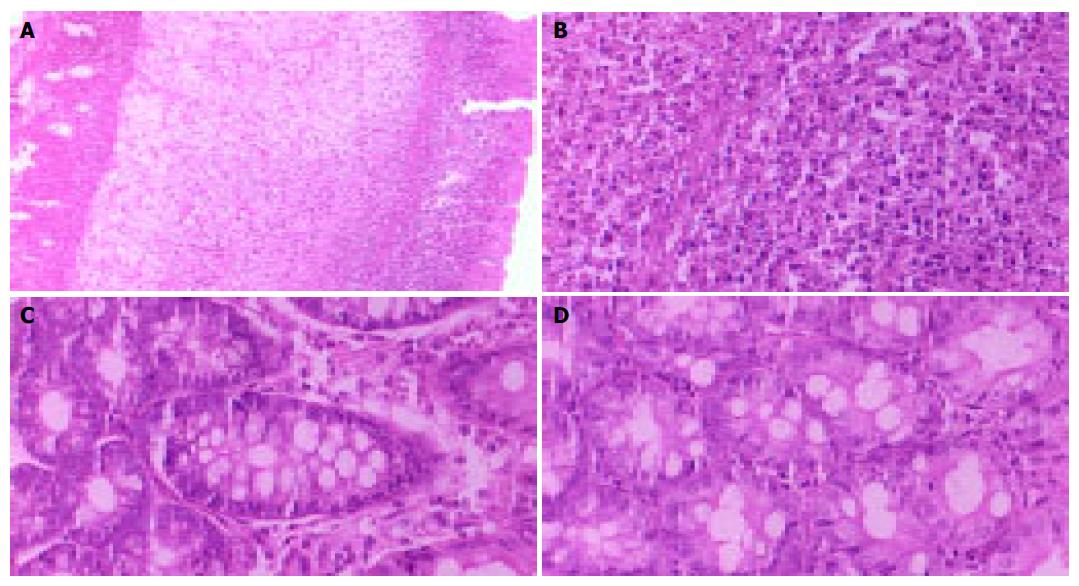
After 3, 7, and 14 d of TNBS administration, macroscopic and histological examination of the rat colon showed that TNBS-induced colitis was uniform, with submucosal and mucosal infiltration with numerous inflammatory cells, signs of extensive ulceration, and dilated blood vessels (Figures 1A and 1B). The colon appeared macroscopically normal in rats that received TNBS for 28 d (Figure 1C). The presence of inflammatory cells was minimal in the colon of the control rats treated with saline (Figure 1D).
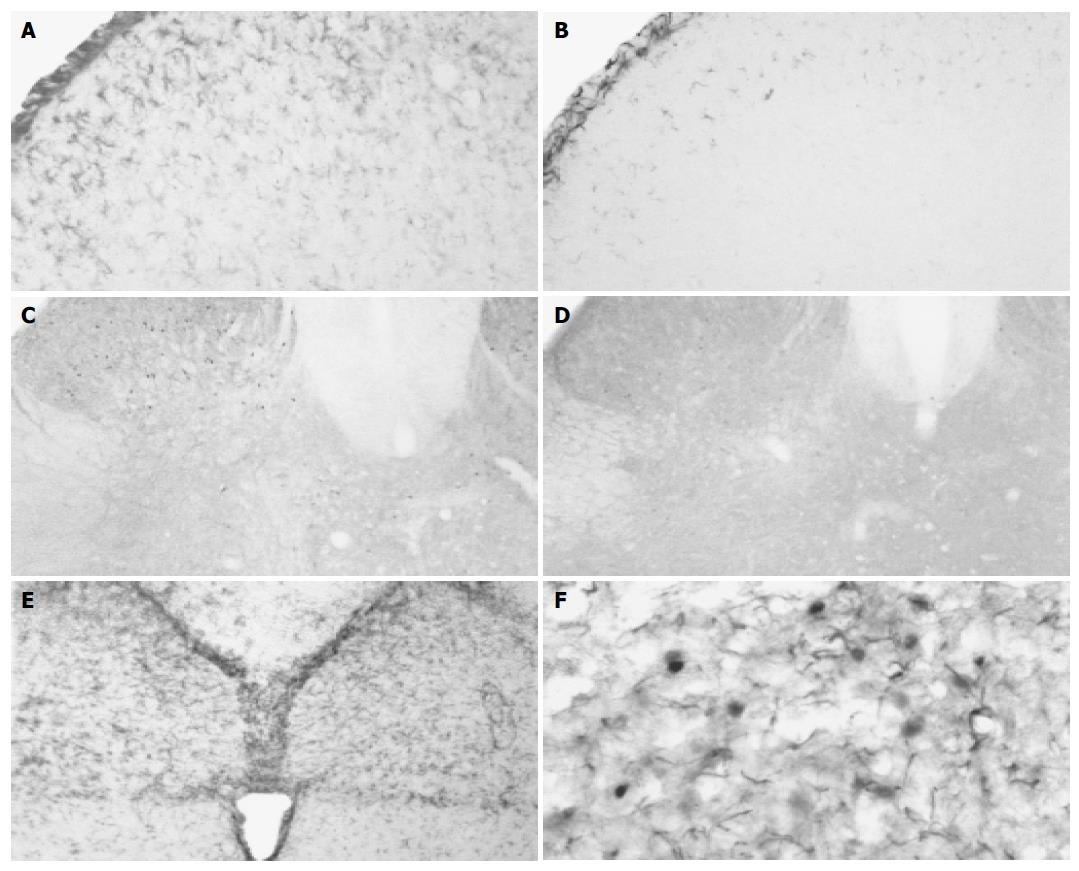
In the experimental animals, colonic inflammation induced robust astrocytic activation responses after 3, 7, and 14 d of TNBS administration. The activated glial cells were characterized by decreased ramification, hypertrophy and proliferation. Most activated astrocytes positive for GFAP were distributed bilaterally in the superficial laminae (laminae I-II) of dorsal horn, intermediolateral nucleus (laminae V), posterior commissural nucleus (laminae X) and anterolateral nucleus (laminae IX, Figure 2A). Mild GFAP-IR astrocytes exhibited in the spinal cord of the rats treated with saline (Figure 2B). The density of GFAP in the spinal cord of experimental rats was significantly higher after 3, 7, and 14 d of TNBS administration compared with that of the controls (P<0.05). After 28 d of TNBS administration, the density of GFAP in the spinal cord decreased and was comparable to that of the control group (P>0.05, Table 1).
The majority of Fos-IR neurons were distributed bilaterally in L5, L6, and S1 segments of lumbo-sacral spinal cord after TNBS administration. At various post-inflammatory days, Fos-IR cells were mainly localized in deeper laminae III-IV and laminae V-VI in the spinal dorsal horn (Figure 2C). Only a few Fos-IR neurons were sparsely distributed in the dorsal horn of the rats treated with saline (Figure 2D). After 3 d following TNBS administration, the number of Fos-IR neurons in the dorsal horn did not increase significantly (P>0.05). With the aggravation of colonic inflammation, the number of Fos-IR cells in the dorsal horn increased after 7 and 14 d following TNBS injection, a significant difference compared with that of the control group (P<0.05). It was noted that Fos expression in the spinal cord displayed two patterns after 28 d of TNBS administration. Few Fos-IR neurons exhibited in the spinal dorsal horn in two rats (12.0 and 13.3, 12.7±0.9), without significant difference from the control (P>0.05). However, many Fos-IR neurons presented in the spinal dorsal horn in three rats (56.7, 69.0 and 36.7, 54.1±16.3), with a significant difference compared with that in control rats (P<0.05, Table 2).
In the control animals, only few GFAP-IR astrocytes exhibited in the medulla oblongata. After TNBS administration, GFAP-IR astrocytes increased and were primarily localized in MVZ, which was composed of NTS, VLM, and IRt, from the level of pyramidal decussation to the level rostral to the obex (Figure 2E). The density of GFAP in MVZ was significantly increased after 3 d of TNBS administration compared with that of the control group (P<0.05). After 7, 14, and 28 d of TNBS administration, the density of GFAP in MVZ decreased gradually and became comparable to the control group (P>0.05, Table 1).
Similarly, Fos-IR neurons were primarily localized in MVZ in the experimental animals. Fos-IR neurons in MVZ did not increase significantly after 3 d of TNBS administration (P>0.05). After 7 and 14 d of TNBS administration, Fos-IR neurons in MVZ increased significantly compared with those in control animals (P<0.05). Fos-IR cells in MVZ decreased significantly and were comparable to those in the control group after 28 days of TNBS administration (41.231.4, P>0.05, Table 2).
In the medulla oblongata, activated astrocytes positive for GFAP enveloped activated neurons stained for Fos (Figure 2F).
It is well known that gut afferent signals reach conscious perception through a three-neuron chain. The second-order neuron, whose cell body is in the dorsal horn of the spinal cord, converge visceral and somatic afferents[6]. In the rat, the descending colon is innervated by sensory afferent fibers in the pelvic nerve projecting to the lumbar-sacral (L6-S2) spinal cord[7]. MVZ is an arch zone extending from the dorsomedial area across reticular formation to VLM. It is situated in the middle-caudal segment of the medulla oblongata, from the level of the pyramidal decussation to the level, rostral to the obex. MVZ is a relay station predominantly transmitting visceral sensory information, and takes part in perception of noxious stimulation[8]. Because of the importance of the lumbo-sacral spinal cord segments and MVZ in transmitting visceral signals, we studied the responses of astrocytes and neurons induced by colonic inflammation in these tissues.
Studies have shown that astrocytes may respond to a number of stimulations, such as somatic and visceral inflammation[3,9], nerve injuries[10] and bone cancer[11]. Activated astrocytes may involve in mediating hyperalgesia and chronic pain. The activated astrocytes are characterized by decreased ramification, hypertrophy, proliferation, and the up-regulation of immunoregulatory molecules[12]. GFAP are distributed in the cytoplasm of many types of glial cells. GFAP expression is low in the intact adult CNS, but increases after astrocytic activation[13]. Sweitzer et al., reported that acute peripheral inflammation induced by subcutaneous injection of formalin and zymosan resulted in increased expression of GFAP in astrocytes. Astrocytic activation has been implicated in regenerative processes and synaptic remodeling that may be detrimental when aberrant processing ensues, leading to chronic pain states[1].
In our experiment, colonic inflammation induced robust astrocytic activation responses after 3, 7, and 14 d of TNBS administration. Most activated astrocytes positive for GFAP were distributed bilaterally in the superficial laminae (laminae I-II) of dorsal horn. These results are consistent with other studies. In a rat model of bone cancer, significant enhancement of GFAP staining was described in the superficial laminae of the ipsilateral spinal cord[11]. Watkins et al., have shown that astrocytes were able to release nerve growth factor (NGF), which exerts its pain modulatory actions on primary afferents. Furthermore, primary afferents expressing high affinity NGF receptors terminate exclusively in laminae I and the outer region of laminae II. Astrocytes in the intermediolateral nucleus (laminae V), posterior commissural nucleus (laminae X) and anterolateral nucleus (laminae IX) were also activated after 3, 7, and 14 d of TNBS administration. In the lumbo-sacral spinal cord, cell bodies of parasympathetic nerve are located in intermediolateral nucleus i.e., is parasympathetic sacral nucleus. Somatic and visceral afferents converge at posterior commissural nucleus. Therefore, astrocytic responses in these nuclei were directly activated by colonic inflammation. Astrocytic activation in anterolateral nucleus might be related to diarrhea induced by TNBS administration.
The timing of the changes of GFAP is relevant to the mode by which astrocytes are activated. A rapid increase in GFAP-IR has been reported to occur between 30 min and 3 h and then a secondary increase which follows approximately 3 d later. GFAP-IR in the trigeminal ganglion in response to dental injury reported by Stephenson et al., appeared by 3 d and was most intense by 7 d[4]. Astrocytic activation in the spinal cord segments increased 42 d after lumbar root injury[14]. Chudler et al., proposed that the persistence of GFAP-IR 59 d after nerve injury suggested GFAP was involved in the long-term recovery of injured neurons[15]. In the experimental rats, the density of GFAP in lumbo-sacral spinal cord and MVZ was most intense by 3 d and decreased gradually after TNBS administration. After 7 and 14 d of TNBS administration, the density of GFAP in the spinal cord was still significantly higher compared with that of the controls (P<0.05). However, the density of GFAP in MVZ became comparable to that of the control group (P>0.05). This result suggested that astrocytic activation in the spinal cord lasted much longer than that in MVZ. The response of astrocytes in both the spinal cord and MVZ decreased significantly with the recovery of colonic inflammation.
The evoked expression of the immediate-early-gene-encoded protein, Fos, serves as a quantifiable marker to identify neuronal populations activated by noxious somatic and visceral stimulation[16]. Fos protein is thought to be a nuclear third messenger coupling short-term extracellular signals with long-term alterations in cell function[17]. Thus, Fos protein expression can be used to study long-term changes in stimulus-induced neuronal activity[18]. We found that Fos-IR cells were mainly localized in deeper laminae III-IV and laminae V-VI in the spinal dorsal horn over time in experimental groups. Only a few Fos-IR neurons were sparsely distributed in the superficial laminae I-II in the dorsal horn. This suggested that persistent noxious visceral stimulation induced Fos expression to a greater extent in deeper laminae than in the superficial ones in the spinal dorsal horn. These results are in line with the spatial distribution of Fos protein in the lumbar spinal dorsal horn in rats following chronic adjuvant-induced arthritis[19] and chronic constriction injury to the sciatic nerve[20]. The study of Imbe et al., showed that Fos-IR cells might spread from the superficial laminae to deeper ones in the dorsal horn when deep tissue inflammation persisted[18].
The timing changes of Fos expression differed from that of GFAP. After 3 d following TNBS administration, the number of Fos-IR neurons in the spinal dorsal horn and MVZ did not increase significantly. With intensification of colonic inflammation, the number of Fos-IR cells both in the dorsal horn and in MVZ increased significantly after 7 and 14 d following TNBS administration. After 28 d of TNBS administration when colonic inflammation recovered, Fos-IR cells in MVZ decreased significantly and were comparable to those in the control group. However, a lot of Fos-IR neurons still presented in the spinal dorsal horn in three experimental rats. Although it is not known exactly whether increased central hyperexcitability contributes to greater Fos protein expression following inflammation. The study of Zimmerman and Herdegen suggested that the increase in Fos may be a necessary pre-requisite for the development of chronic pain and allodynia, because increased expression of the gene product renders the neurons susceptible to subsequent stimuli[21]. The development of neuronal activation in the lumbo-sacral dorsal horn may play a role in visceral hypersensitivity.
Astrocytes display an intimate structural relationship with the neurons that they envelope in the CNS. Gap junctions between astrocytes have been demonstrated using the freeze-fracture technique and adherents junctions between neurons and astrocytes have been observed in many species[4]. In addition to the morphological associations between neurons and astrocytes, there appears to be a wide range of functional interactions between these cells. Duan et al., reported that the number of connexin32/connexin43 gap junction (HGJ) between neurons and astrocytes in rat supraoptic nuclei was significantly increased following hyperosmotic stimuli. They proposed HGJ may be a rapid adaptive signal structure between neurons and astrocytes in response to stimulation. Apart from playing roles in synaptic remodeling and providing neurotrophic support for regenerating neurons, astrocytes which are known to express both voltage gated sodium channels and glutamate receptors could serve as sensors for neuronal activity or injury[22]. The present study has shown that GFAP-IR astrocytes and Fos-IR neurons distributed similarly in deeper laminae of the spinal dorsal horn and MVZ, especially in the commissural subnucleus inside NTS. This suggested that colonic inflammation could not only induce neuronal activation in visceral transmitting chain, but also induce astrocytic responses via signal interactions between neurons and astrocytes.
| 1. | Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 264] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002;956:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Qiu JY, Duan L, Chen LW, Rao ZR. Expression of GFAP in rat brain stem astrocytes induced by stomachic nociception and relationship to neurons. Zhongguo Zuzhi Huaxue He Xibao Huaxue Zazhi. 2001;10:219-223. |
| 4. | Stephenson JL, Byers MR. GFAP immunoreactivity in trigeminal ganglion satellite cells after tooth injury in rats. Exp Neurol. 1995;131:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Zhou SY, Mei QB, Liu L, Guo X, Qiu BS, Zhao DH, Cho CH. Delivery of glucocorticoid conjugate in rat gastrointestinal tract and its treatment for ulcerative colitis. Acta Pharmacol Sin. 2001;22:761-764. [PubMed] |
| 6. | Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut. 2001;48:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Rao ZR, Ju G. Morphology of the medullary visceral zone. Chinese Science Bulletin. 1999;44:1-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 277] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp Neurol. 1999;157:289-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 351] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O'Reilly T, Wotherspoon G. A rat model of bone cancer pain. Pain. 2002;96:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Ramer MS, Kawaja MD, Henderson JT, Roder JC, Bisby MA. Glial overexpression of NGF enhances neuropathic pain and adrenergic sprouting into DRG following chronic sciatic constriction in mice. Neurosci Lett. 1998;251:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine (Phila Pa 1976). 2000;25:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 208] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Chudler EH, Anderson LC, Byers MR. Trigeminal ganglion neuronal activity and glial fibrillary acidic protein immunoreactivity after inferior alveolar nerve crush in the adult rat. Pain. 1997;73:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Dai Y, Iwata K, Kondo E, Morimoto T, Noguchi K. A selective increase in Fos expression in spinal dorsal horn neurons following graded thermal stimulation in rats with experimental mononeuropathy. Pain. 2001;90:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Abbadie C, Besson JM. C-fos expression in rat lumbar spinal cord following peripheral stimulation in adjuvant-induced arthritic and normal rats. Brain Res. 1993;607:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yamazaki Y, Maeda T, Someya G, Wakisaka S. Temporal and spatial distribution of Fos protein in the lumbar spinal dorsal horn neurons in the rat with chronic constriction injury to the sciatic nerve. Brain Res. 2001;914:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Zimmerman M, Herdegen T. Plasticity of the nervous system at the systemic, cellular and molecular levels: a mechanism of chronic pain and hyperalgesia. Prog Brain Res. 1996;110:233-259. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 283] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Science Editor Zhu LH and Guo SY Language Editor Elsevier HK