Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4689
Revised: November 1, 2004
Accepted: November 4, 2004
Published online: August 14, 2005
AIM: To investigate the role of survivin expression in the pathogenesis of colorectal carcinoma.
METHODS: Immunohistochemistry S-P method and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) were used to detect the expression of survivin and apoptotic cell in situ in colorectal cancerous tissues, para-cancerous tissues and normal tissues of 48 cases of colorectal carcinoma.
RESULTS: The survivin positive unit (PU) was higher in cancerous tissues (38.76 ± 5.14) than in para-cancerous (25.17 ± 7.26) or normal tissues (0.57 ± 0.03) (P < 0.05). The apoptosis index (AI) of para-cancerous tissues was (7.51 ± 2.63%) higher than cancerous tissues (4.65 ± 1.76%). The expression of survivin was associated with pathological grade, lymph node metastasis and Dukes stage of colorectal carcinoma.
CONCLUSION: Survivin expression may play an important role in carcinogenesis of colorectal carcinoma and may be associated with malignant biological behaviors of colorectal carcinoma.
- Citation: Tan HY, Liu J, Wu SM, Luo HS. Expression of a novel apoptosis inhibitor-survivin in colorectal carcinoma. World J Gastroenterol 2005; 11(30): 4689-4692
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4689
Survivin is a new member of inhibitors of apoptosis proteins (IAP) gene family that has been found recently. The surviving gene lies in the 17q25 of human chromosome with unique structure and characteristics, coding a 16.5 KD protein[1]. Survivin protein contains only one BIR (baculovirus IAP repeat) domain and does not have the zinc-binding fold terminated with carboxyl. Furthermore, under normal circumstances survivin is expressed in embryonic and fetal tissues, but completely downregulated in normal adult tissues. Interestingly, this protein is found to be prominently reexpressed in a variety of human malignant transformation cell lines and tumorous tissues[2,3]. And its unique structure and biological function have interested so many scholars during their researches on the molecular biology of tumors.
Colorectal carcinoma has a high incidence in China. The deficiency of cell apoptosis plays an important role in the pathogenesis of this carcinoma. It is still uncertain about the role of survivin expression in colorectal carcinoma. In the present study, the expression of survivin and cell apoptosis were detected. The correlation between survivin expression and cell apoptosis and the role of survivin in the pathogenesis of colorectal carcinoma were also investigated.
Forty-eight cases of colorectal carcinoma who received rectetomy were obtained from the Department of General Surgery, Renmin Hospital of Wuhan University. Twenty-six of them were male and 22 female. The mean age was 55.8 years ranged from 37 to 75 years. All the cases did not receive radiation treatment or chemotherapy before surgery and had been diagnosed by two doctors at the department of pathology. Three pieces of tissues were taken respectively from cancerous tissues, para-cancerous tissues (5 cm away from cancerous tissues) and normal tissues (10 cm away from cancerous tissues). All the specimens were fixed in 10% neutral-buffered formalin, dehydrated in ascending series of ethanol and routinely embedded in paraplast. Sections were cut at 4 μm, stained with hematoxylin and eosin for histopathological and immunohistochemical evaluation as well as TUNEL. The clinicopathological parameters are summarized in Table 1.
| Subjects | Clinical pathological index | n | Survivin PU | P |
| Gender | Male | 26 | 42.95 ± 16.87 | P > 0.05 |
| Female | 22 | 30.96 ± 16.06 | ||
| Age (yr) | < 55 | 14 | 36.95 ± 19.86 | P > 0.05 |
| 55 | 34 | 38.96 ± 15.37 | ||
| Tumor | < 3 cm | 14 | 36.15 ± 16.29 | P > 0.05 |
| Diameter | 3 cm | 34 | 38.28 ± 18.16 | |
| Pathological | High and intermedium | 24 | 33.16 ± 14.28 | P < 0.05 |
| differentiated | ||||
| Grade | Cannular adenocarcinoma | |||
| Low differentiated tubular | 10 | 51.93 ± 20.89 | ||
| adenocarcinoma | ||||
| Other types | 14 | 35.61 ± 17.22 | ||
| Lymph node | Negative | 26 | 30.12 ± 13.33 | P < 0.05 |
| Positive | 22 | 56.21 ± 11.95 | ||
| Dukes stage | A | 16 | 20.16 ± 5.16 | P < 0.05 |
| B | 4 | 24.85 ± 3.12 | A/B Stage | |
| C | 10 | 45.13 ± 10.21 | VS | |
| D | 14 | 59.66 ± 10.21 | C/D stage |
All the specimens were incubated in 3% hydrogen peroxide for 15 min to inactivate the endogenous peroxidase and then heated in 0.01 mol/L citrate buffer for antigen retrieval through 12 min microwave pre-treatment. Incubated with 10% goat serum, all the specimens were subsequently reacted with rabbit-anti-human survivin polyclonal antibody (Neomarkers, USA, 1:1000 dilution) at 4°C overnight. Immunohistological staining was performed according to SP detection kit.
Apoptotic cells were detected, according to the procedure recommended by in situ cell apoptosis detection kit (Boehringer Mannheim Company, Germany).
Immunohistochemical quantitative evaluation All the analyses were performed using the HIPAS-2000 computer image analysis system (produced by Tongji Qianping Image Engineering Company) Images were captured at ×400 magnification by micrographic system . Image analysis system separated staining positive area from background. Then the gray level units as well as areas of positive staining and background could be measured. According to Shen’s method, positive unit represents the relative concentration of positive staining[4]. Each section was observed randomly at five areas and the mean PU was calculated.
The apoptotic cells were located sporadically with nuclei stained yellow or brownish yellow. A mean percentage of positive cells among 500 cells was determined in five areas at ×400 magnification. The results could be recorded as apoptosis index (AI).
All analyses were performed with t test and ANOVA using SPSS 9.0 software (Statistical Package for Social Science). P values < 0.05 were considered to indicate statistical significance.
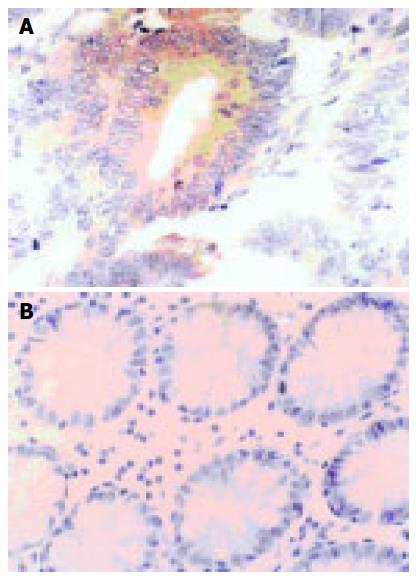
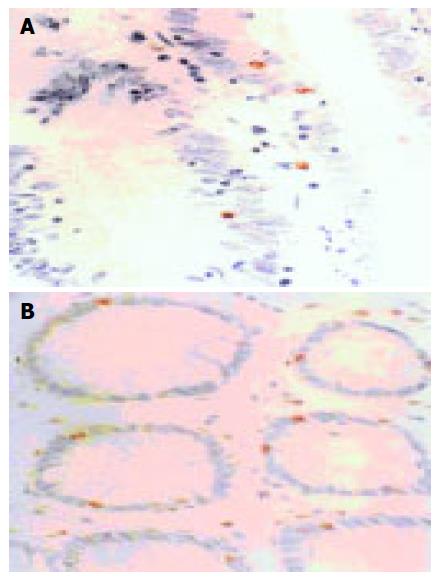
Survivin PU were mainly in the cytoplasm of para-cancerous or cancer cells. The nuclei could be stained equally light yellow or brownish yellow, located sporadically or in the form of sheets (Figure 1A). Survivin PU was rarely expressed in normal large intestinal mucosa (Figure 1B). Quantitative analysis of immunohistochemistry is summarized in Table 2. The apoptotic cells were distributed sporadically or in clusters with brownish yellow nuclei. apoptotic cells were found to be rare and weak stained in normal large intestinal mucosa, most of which were located in epithelium (Figure 2A), but scattered sporadically in cancerous (Figure 2B) and para-cancerous tissues. Survivin PU in cancerous tissues 38.76 ± 5.14 was significantly higher than in para-cancerous 25.17 ± 7.26 and normal tissues 0.57 ± 0.03 (P < 0.05). And the AI in para-cancerous tissues, (7.51 ± 2.63)% was significantly higher than in cancerous tissues, (4.65 ± 1.76)%(P = 0.0075).
Survivin PU was not correlated with sex, age and tumor diameter of patients, but correlated with pathological grade, lymph node and Dukes stage. Survivin PU in cancerous tissues with low differentiation, lymph node positive and Dukes C/D stage was higher than in cancerous tissues with high differentiation, lymph node negative, and Dukes A/B stage.
According to recent clinical and statistical data, there was a gradually increasing incidence in colorectal carcinoma. carcinogenesis can be regarded as a complex process with multi-gene participation and multi-steps. As we all know, most colorectal carcinomas originate from adenoma. Abnormalities in the control of programmed cell death (apoptosis) play an important role in the pathogenesis of colorectal carcinoma during the process from adenoma to cancer. It has been proved that the genes closely related with the control of colorectal cell apoptosis include bcl-2, c-myc, p53 and IAP gene family. So the research and application of those apoptosis-related genes is of great importance in the diagnosis, treatment and prognosis judgement of colorectal carcinoma.
Survivin is a new member of IAP gene family, which was obtained by Altieri in Yale University through hybridization and filtration of human genome[5]. It can combine with microtubules of mitotic spindle,and through interaction with caspases it can inhibit cell apoptosis[6]. Survivin affects various terminal effect factors and might be one of the strongest apoptosis inhibitory factors till now. This research using immunohistochemical staining showed that survivin was very weak in normal epithelial cells and partially expressed in para-cancerous tissues. But positive cells in para-cancerous tissues were less than in cancerous tissues with a weaker staining, suggesting that survivin expression could be an early event during the pathogenesis of colorectal carcinoma. This is in accordance with the researches on pancreatic and hepatocellular cancers. Evidence showed that survivin could be expressed in early stage of pancreatic cancer or prec-ancerous lesions[6,7]. Sarela found survivin mRNA mainly existed in survivin positive cancerous tissues, but hardly in survivin negative cancerous tissues[8]. Thus, it is proposed that, survivin is an oncogene, and also a marker with great potential for tumor diagnosis. Survivin antibody could also be taken as a common marker for early diagnosis of colorectal carcinoma[9,10].
This research proved that survivin PU had no significant correlation with sex, age, and tumor diameter, but correlated with differentiation stage, lymph node and Dukes stage of colorectal carcinoma. Survivin PU in cancerous tissues with low differentiation, lymph node positive and Dukes C/D stage was higher than in cancerous tissues with high differentiation, lymph node negative and Dukes A/B stage. It indicated that survivin PU was related with malignant biological behaviors. Its continuous expression might be associated with the development of malignant tumor. Researches on gastric carcinoma, lung cancer, breast cancer revealed that survivin PU was not only correlated with malignant biological behaviors such as invasion, metastasis, etc, but also correlated with recurrence, reduced survival time after surgery. It might be taken as an independent index for judging the prognosis[11-13]. Therefore, survivin is of great value for diagnosis and prognosis judgement of malignant tumors.
Survivin has the feature of selective expression in various malignant tumors, which might be essential for carcinogenesis of those tumors. But the mechanism for survivin to contribute to carcinogenesis of tumors is not clear yet. It is probably involved in cell apoptosis, proliferation, etc. Our research showed that the apoptotic cells mainly located in epithelium of normal large intestinal mucosa, but distributed sporadically in cancerous and para-cancerous tissues. AI of paracancerous tissues was higher than cancerous tissues, indicating that survivin could inhibit apoptosis of colorectal carcinoma cells. This might be regarded as part of mechanisms for its participation in carcinogenesis of colorectal carcinoma. But the precise pathway for survivin to inhibit apoptosis still needs further investigation. According to the present study, survivin had various pathways to inhibit apoptosis, which could also be found in other malignant tumors. It might play an important role in the carcinogenesis of various tumors.
| 1. | Reed JC. The Survivin saga goes in vivo. J Clin Invest. 2001;108:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Adida C, Crotty PL, McGrath J, Berrebi D, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43-49. [PubMed] |
| 3. | Xu Y, Fang F, Ludewig G, Jones G, Jones D. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in cancer cells. DNA Cell Biol. 2004;23:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Hong S. Research on quantative methods of immunihi-stochemical staining. Zhongguo Zuzhi Huaxue Yu Xibaohuaxue Zazhi. 1995;4:89-91. |
| 5. | Altieri DC. Molecular cloning of effector cell protease receptor-1, a novel cell surface receptor for the protease factor Xa. J Biol Chem. 1994;269:3139-3142. [PubMed] |
| 6. | Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136-143. [PubMed] |
| 10. | Yagihashi A, Asanuma K, Nakamura M, Araya J, Mano Y, Torigoe T, Kobayashi D, Watanabe N. Detection of anti-survivin antibody in gastrointestinal cancer patients. Clin Chem. 2001;47:1729-1731. [PubMed] |
| 11. | Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315-5320. [PubMed] |
| 12. | Span PN, Sweep FC, Wiegerinck ET, Tjan-Heijnen VC, Manders P, Beex LV, de Kok JB. Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem. 2004;50:1986-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Johnson ME, Howerth EW. Survivin: a bifunctional inhibitor of apoptosis protein. Vet Pathol. 2004;41:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
Science Editor Zhu LH and Guo SY Language Editor Elsevier HK