Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4596
Revised: January 10, 2005
Accepted: January 14, 2005
Published online: August 7, 2005
AIM: To investigate the effects of polo-like kinase-1 (PLK1) antisense phosphorothioate oligodeoxynucleotide (ASODN) on apoptosis and cell cycle of human colon cancer cell line SW480.
METHODS: After SW480 colon cancer cells were transfected with PLK1 ASODN, Northern and Western blot analyses were used to examine PLK1 gene expression in cancer cells. We studied apoptosis using terminal uridine deoxynucleotidyl nick end labeling. Apoptosis and cell cycle of SW480 cells were examined by fluorescence-activated cell sorter scan.
RESULTS: The levels of PLK1 mRNA and protein were greatly inhibited by PLK1 ASODN in SW480 cancer cells transfected with PLK1 ASODN. Apoptosis index (AI) induced PLK1 ASODN in a time- and dose-dependent manner. Results from FLM showed that sub-2N DNA content of transfected cancer cells was significantly increased and arrested at G2/M compared with control groups.
CONCLUSION: PLK1 ASODN can induce apoptosis of human colon cancer cell line SW480.
- Citation: Fan Y, Zheng S, Xu ZF, Ding JY. Apoptosis induction with polo-like kinase-1 antisense phosph-orothioate oligodeoxynucleotide of colon cancer cell line SW480. World J Gastroenterol 2005; 11(29): 4596-4599
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4596.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4596
The polo kinase family includes mammalian polo-like kinase (PLK) 1, Snk, Fnk, Xenopus laevis Plx1, Drosophila polo, fission yeast PLO1, and budding yeast Cdc5[1]. Genetic and biochemical experiments in various organisms indicate that PLK are important regulators of many cell-cycle-related events, including activation of Cdc2, chromosome segregation, centrosome maturation, bipolar spindle formation, regulation of anaphase-promoting complex, and execution of cytok-inesis[1,2]. The importance of PLK1 as a measure for the aggressiveness of a tumor seems to result from its different functions during mitotic progression in particular, its role in the G2/M transition (phosphorylation of cyclin B1, a component of the mitosis-promoting factor)[3-5]. PLK1 also phosphorylates substrates that are involved in several additional steps of mitotic progression, including components of the anaphase-promoting complex and the cytokinesis machinery[6,7]. The activity of PLK1 is elevated in tissues and cells with a high mitotic index, including cancer cells[8,9]. An increasing body of evidence suggests that the level of PLK1 expression has prognostic value for predicting outcomes in patients with different cancers, including non-small cell lung cancer, squamous cell carcinoma of the head and neck, esophageal carcinoma, oropharyngeal carcinoma, ovarian cancer, breast cancer, endometrial carcinoma, colorectal cancer, and glioma[10-18]. Takahashi et al[18] found that some crypt cells in normal colon mucosa show weakly positive staining for PLK1, and PLK1 overexpresses in most of colorectal cancers. Moreover, PLK1 expression is associated with pT (primary tumor invasion) (P<0.0006), pN (regional lymph nodes, P<0.008) and the Dukes’ classification (P<0.0005). The results suggest that overexpression of PLK1 might be of pathogenic, prognostic and proliferative importance, so that this kinase might have potential as an invasion and metastasis marker for colorectal cancer. But whether PLK1 plays a role in colon cancer cell is not clear.
In this article, we used ASODN to inhibit the expression of PLK1 in human cancer cell lines SW480 and determined the role of PLK1 in tumorigenesis.
New fetal calf serum, RPMI-1640, phosphate-buffered saline (PBS), oligofectamine, penicillin and streptomycin, and trypsin were obtained from Invitrogen (Karlsruhe, Germany). Colon cancer line SW480 was obtained from the Institute of Cell Biology, Shanghai, China. Cells were maintained in RPMI 1640 supplemented with 10% newborn bovine serum at 37 °C in 50 mL/L CO2 atmosphere. The sequence of antisense phosphorothioate oligodeoxynucleotide (ASODN) target PLK1 was 5’-GCAGACCTCGATCC-GAGCAG-3’, 20-mer[20].
Cancer cells were transfected with ASODN using the oligofectamine protocol (Invitrogen). In brief, 1 d prior to transfection, cancer cells were seeded without antibiotics onto 24-well plates, 1105 cells/well, corresponding to a density of 40-50% at the time of transfection. Cancer cells were transfected with ASODN at different doses (25, 50, 100 nmol/L). There were two control groups: control cells were incubated with RPMI 1640 alone without ASODN or oligofectamine. Cells were harvested 12, 24, 48, and 72 h after the transfection. All transfections were performed in triplicate at each time point.
At 12, 24, 26, 48, 72 h after transfection, total RNAs was isolated using TRIzol according to the manufacturer’s protocol (Invitrogen). 18 and 28 S were used as an inner standard. Probes for Northern blots were generated by radiolabeling using 250 μCi of [α-32 P] dCTP (6 000 Ci/mmol) according to the Prime-a-gene labeling System’s protocol for each reaction. Northern blotting and hybridizations were carried out routinely.
Protein level was determined with Western blot. At 12, 24, 26, 48, and 72 h after transfection, total protein of cancer cells of controls and each transfected group was extracted and protein concentration was determined. Western blot assay was performed with routine methods.
Terminal uridine deoxynucleotidyl nick end labeling (TUNEL) assay was performed by using the in situ cell detection kit (FITC) following the manufacturer’s instructions (Roche Molecular Biochemicals). In brief, cells grown on glass coverslips were fixed by a freshly prepared paraform-aldehyde solution (4% in PBS, pH 7.4) for 1 h at room temperature. Coverslips were then washed with PBS and incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 min in ice. Then, 50 μL of TUNEL reaction mixture was added on coverslips and incubated in a humidified chamber for 1 h at 37 °C in the dark. Finally, cells were mounted and examined by microscopy as described above. TUNEL-positive (apoptotic) cells were stained bright green. Apoptosis index (AI) was calculated by apoptosis cells/total cell100%.
Cell cycle distribution and apoptosis were analyzed using a fluorescence-activated cell sorter (FACS) scan apparatus (BD Biosciences, Heidelberg, Germany). For the determination of cell cycle distribution, cells were harvested, washed with PBS and probed with CycleTESTTM PLUS DNA reagent kit (BD Biosciences) according to the manufacturer’s protocol. For each transfection (control and different doses of ASODN), 30 000 cells were analyzed in triplicate. The percentage of cells in different cell cycle phases was calculated using ModFit LT for Mac (BD Biosciences). For the detection of apoptotic phenotypes, harvested cells were fixed with ice-cold 70% ethanol and treated for 20 min at 37 °C with RNase A at 5 μg/mL and propidium iodide (PI) at 50 μg/mL. Subsequent analyses of cell cycle distribution and apoptosis were performed using CELLQuest software (BD Biosciences).
SPSS 10.0 was performed to assay the data. Data were analyzed using SPSS. P<0.05 was considered statistically significant.
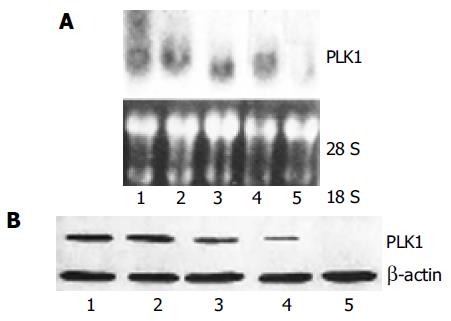
We first tested the ability of ASODN to reduce the endogenous level of PLK1 mRNA and protein in the SW480 cancer cell line. PLK1 mRNA and protein levels in untransfected control cells were not significantly influenced. Transfection with PLK1 ASODN at a concentration of 50 nmol/L led to a statistically significant loss of PLK1 mRNA at 12 h after the beginning of transfection (Figure 1). We found that SW480 cancer cells transfected with ASODN showed a statistically significant reduction in PLK1 protein levels 24 h after ASODN transfection as compared with untransfected control cells. The results showed that ASODN could exert inhibitory effects on mRNA and protein expression of PLK1 gene in SW480 cancer cells at 50 nmol/L.
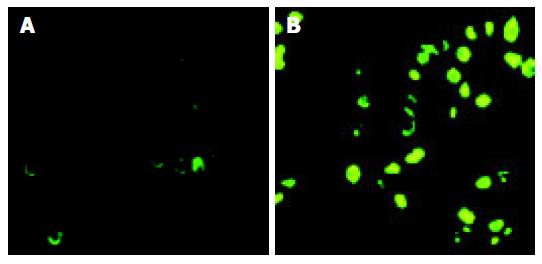
As shown in Figure 2, apoptosis was observed in cancer cells transfected with PLK1 ASODN. AI increased in a time- and dose-dependent manner. These data suggested that ASODN could induce apoptosis of colon cancer cell line SW480.
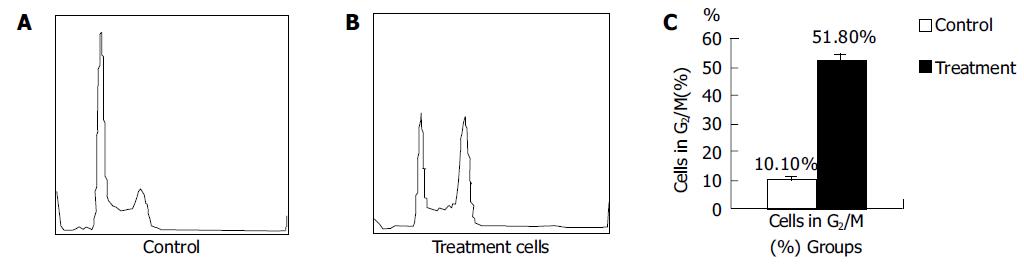
To study the apoptosis induced by ASODN, we investigated whether PLK1 levels were associated with apoptosis in all cancer cell lines by determining the sub-2N DNA content with FACScan analysis. Untransfected cells contained 1.9% cells with sub-2N DNA, transfection with ASODN was associated with 16.5% sub-2N DNA content in SW-480 cells. Next, we analyzed the effect of PLK1 depletion on cell-cycle progression using FACScan. As shown in Figure 3, no substantial change in cell cycle distribution was detected in control (untransfected or oligofectamine-treated alone) cells. Transfection of PLK1 ASODN induced significant G2/M arrest 48 h after transfection with 50 nmol/L ASODN: SW-480 cells showed a fivefold increase in the percentage of cells in G2/M compared to untransfected cancer cells. These data suggested that ASODN could induce apoptosis and G2/M arrest in human colon cancer cell line SW480.
The polo family of protein kinases is an important group of cell cycle regulators[1]. PLK1 is one of mammalian members of this gene family. In lower organisms, members of the polo family have been shown to control centrosomal function, chromosomal segregation, and cytokinesis[8]. A close correlation between mammalian PLK1 expression and carcinogenesis was recently documented. Mammalian PLK1 was found to be overexpressed in various human tumors[10-17]. It has been proposed that PLK1 could be used as a novel diagnostic marker for several types of cancers[9,19]. Furthermore, constitutive expression of PLK1 in NIH 3T3 cells causes oncogenic focus formation and induces tumor growth in nude mice[20]. Therefore, inhibition of PLK1 function is important for cancer therapy.
Immunohistochemical analyses showed that PLK1 is expressed in 78 primary colorectal cancers as well as 15 normal colorectal specimens[18]. In normal colon mucosa, some crypt cells showed weakly positive staining for PLK1 in 13 out of 15 cases, the remaining cases being negative. Elevated expression of PLK1 was observed in 57 (73.1%) of colorectal cancers, which was significantly associated with pT (P<0.0006), pN (P<0.008) and the Dukes classification (P<0.0005). The results suggest overexpression of PLK1 might be of pathogenic, prognostic and proliferative importance, and might have potential as a marker for colorectal cancer. In this study, PLK1 is upregulated in colon cancer cell line SW480, suggesting that PLK1 was overexpressed in colon cancer and may play an important role in colon cancer development and progression.
Antisense oligonucleotides can downregulate some oncogenes associated with malignant tumor, and synthetic oligonucleotides have been currently evaluated in clinical trials for the treatment of cancer, inflammation, and viral diseases[21]. Li et al[19] tested some ASODN, ASODN 9 that is most effective in blocking PLK1 expression in liver carcinoma cell line HepG2. But no studies of PLKl ASODN are available on colon cancer. In the present study, SW480 cancer cells were transfected with ASODN 9. mRNA and protein expression of PLK1 gene could be induced significantly by Western and Northern blot. Our results suggest that transfection of ASODN is efficient in suppressing PLK1 expression in SW480 colon cancer cells.
After PLK1 expression was downregulated, we detected apoptosis of cancer cells by TUNEL assay. We observed that transfection with PLK1 ASODN significantly induced AI in a time- and dose-dependent manner. To further confirm the induction of apoptosis in SW480 cells was through the activation of an apoptotic program, we detected apoptosis program by sub-2N DNA content by flow cytometry. It was found that sub-2N DNA content of cancer cells transfected with ASODN increased significantly compared with untransfected cells. Results from flow cytometry analysis further confirmed that ASODN could induce significant apoptosis in SW480 cells. All these data indicate that downregulation of PLK1 expression with ASODN may induce apoptosis in colon cancer cell line SW480.
Meanwhile, cell viability was significantly decreased in cells after ASODN treatment as compared with untreated controls, suggesting that apoptosis may be related to inhibition of SW480 colon cancer cell line.
In addition, we examined the cell cycle distribution changes in ASODN-transfected SW480 cells by flow cytometry. Forty-eight hours after transfection with ASODN, SW480 cancer cells showed a stronger G2/M arrest compared with control cells.
In conclusion, targeting PLK1 expression using ASODN may hold promise as one of the effective therapies for colon cancer. It is hoped that application of this strategy in clinic may improve the efficacy of treatment of patients with colon cancer.
| 1. | Glover DM, Hagan IM, Tavares AA. Polo-like kinases: a team that plays throughout mitosis. Genes Dev. 1998;12:3777-3787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 349] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 266] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 280] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Yuan J, Eckerdt F, Bereiter-Hahn J, Kurunci-Csacsko E, Kaufmann M, Strebhardt K. Cooperative phosphorylation including the activity of polo-like kinase 1 regulates the subcellular localization of cyclin B1. Oncogene. 2002;21:8282-8292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Qian YW, Erikson E, Taieb FE, Maller JL. The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol Biol Cell. 2001;12:1791-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 409] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 7. | Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 1995;9:1059-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 313] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Golsteyn RM, Schultz SJ, Bartek J, Ziemiecki A, Ried T, Nigg EA. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107:1509-1517. [PubMed] |
| 9. | Holtrich U, Wolf G, Bräuninger A, Karn T, Böhme B, Rübsamen-Waigmann H, Strebhardt K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc Natl Acad Sci USA. 1994;91:1736-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 222] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, Altmannsberger HM, Rübsamen-Waigmann H, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 280] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794-2797. [PubMed] |
| 12. | Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. Int J Cancer. 2000;89:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett. 2001;164:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, Strebhardt K, Bleyl U. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000;196:753-759. [PubMed] |
| 16. | Macmillan JC, Hudson JW, Bull S, Dennis JW, Swallow CJ. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol. 2001;8:729-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Dietzmann K, Kirches E, von Bossanyi K, Mawrin C. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K, Kimura M, Okano Y, Saji S. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Li L, Sheng QW, Wei G, Bing HY, Xiao DH. Antitumor activity of antisense oligonucleotides targed at Plk1 in HepG2 cell in vivo and in vitro. Aizheng. 2001;20:1233-1236. |
| 20. | Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, Ferris DK. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Monteith DK, Levin AA. Synthetic oligonucleotides: the development of antisense therapeutics. Toxicol Pathol. 1999;27:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK