Published online Aug 7, 2005. doi: 10.3748/wjg.v11.i29.4490
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: August 7, 2005
AIM To examine the fetal and neonatal esophagogastric junction region (EGJ) histologically for the presence of an equivalent to adult cardiac mucosa (CM); to study the expression patterns of all cytokeratins (CK) relevant to the EGJ during gestation; to compare the CK profile of the gestational and the adult EGJ; and to determine the degree of development in the adult EGJ histology and CK profile during gestation.
METHODS: Forty-eight fetal autopsy specimens of the EGJ were step-sectioned and stained with hematoxylin and eosin (H&E) to select sections showing the mucosal lining. Immunohistochemistry for CK5, 7, 8, 13, 18, 19, and 20 was performed. Antibody staining was then graded for location, intensity, and degree.
RESULTS: The distal esophagus was lined by simple columnar epithelium from 12-wk gestational age (GA). The proximal part of this segment consisted of mucus-producing epithelium, devoid of parietal cells. CK5 and 13 were present exclusively in multilayered epithelia and CK8, 18, and 19 predominantly in simple columnar epithelium. There were no differences in the frequencies of the co-ordinate CK7+/20+ and the CK7-/20- immunophenotypes between different locations. The prevalence of the CK7+/20- immunophenotype decreased, and that of the CK7-/20+ immunophenotype increased significantly from the distal esophagus to the distal stomach.
CONCLUSION: Fetal columnar-lined lower esophagus (fetal CLE) may be the equivalent and precursor of the short segments of columnar epithelium found in the distal esophagus of some normal adult subjects. Esophageal simple columnar epithelium without parietal cells (ESN) may be the precursor of adult CM. The similarities between the fetal and adult EGJ and stomach CK expression patterns support the conclusion that adult CM has an identifiable precursor in the fetus. This would then indicate that at least a part of the adult CM has a congenital origin.
- Citation: Hertogh GD, Eyken PV, Ectors N, Geboes K. On the origin of cardiac mucosa: A histological and immunohistoc-hemical study of cytokeratin expression patterns in the developing esophagogastric junction region and stomach. World J Gastroenterol 2005; 11(29): 4490-4496
- URL: https://www.wjgnet.com/1007-9327/full/v11/i29/4490.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i29.4490
Since the mid-1970s, there has been a continuing increase in the incidence of gastric cardiac adenocarcinoma in the USA and other Western countries[1]. This increase is probably genuine, because it cannot be explained by improved diagnostic methods or classification changes[2]. The pathogenesis of gastric cardiac adenocarcinoma remains unclear. These tumors may arise from foci of intestinal metaplasia, secondary to inflammation of the gastric cardia (carditis)[3]. It is controversial, however, whether factors like gastroesophageal reflux disease, Helicobacter pylori infection or others contribute to the development of inflammation and intestinal metaplasia in the cardia region[4,5]. Research on this topic is hampered by the limited knowledge of the normal histology of the esophagogastric junction region (EGJ). There is no consensus on the normal histology, location, and length of cardiac mucosa (CM). Histological investigation of hematoxylin and eosin (H&E)-stained sections of the EGJ in adults and children has led some investigators to suggest that CM is actually a metaplasia[6-9]. Others, however, support the concept that CM is present from birth as a normal structure[10-14].
Immunohistochemical investigation of the cytokeratin (CK) profile in the EGJ can be used for the study of the origin of adult CM. The keratins are the largest group of intermediate filament proteins expressed by epithelial cells. There are at least 30 different keratins, which are divided into two types. CK filaments are obligate heteropolymers composed of type I and type II proteins. Keratins are typically expressed as pairs in a tissue- and differentiation-specific manner. In 1999, Ormsby et al[15], described a CK7 and 20 immunoreactivity pattern typical for intestinal metaplasia in long-segment Barrett’s esophagus (LSBE). This pattern consists of superficial CK20 staining and strong CK7 staining of both superficial and deep glands. It has been claimed by the same group that Barrett’s CK7/20 pattern also identifies a subset of patients with suspected short-segment Barrett’s esophagus (SSBE) who have a patient profile similar to that seen in LSBE[16]. Glickman et al[17], reported that intestinal metaplasia of the EGJ shows a similar pattern of CK7/20 reactivity as LSBE and SSBE. They also found that non-intestinalized CM was diffusely positive for CK7 and showed surface positivity only for CK20, irrespective of its location in LSBE, SSBE or in the most proximal portion of the stomach. This CK7/20 staining pattern was clearly different from that seen in the normal antrum. According to these authors, the results suggest that CM, whether present in the esophagus or at the EGJ, may already represent a form of metaplastic epithelium. DeMeester et al[18], found the Barrett’s CK7/20 pattern in 85% of CM biopsies. On the basis of these and other findings, they conclude that there is a strong evidence but no conclusive proof that all CM is acquired.
We have earlier done a histological study on mucosal development in the EGJ of embryos and fetuses[19]. In this study, CM was defined as a structure composed of columnar foveolar and surface epithelium overlying glandular structures containing no parietal cells. We found that CM develops during pregnancy and we concluded that it is present at birth as a normal structure. The description of the Barrett’s CK7/20 pattern in adult CM and the fact that this finding has been used to claim a metaplastic origin for CM have urged us to examine our material for the presence of Barrett’s CK7/20 pattern. In addition, we studied the expression patterns of all other CKs relevant to the EGJ during gestation.
The study material was derived from 48 embryonic, fetal and neonatal autopsies performed between August 1999 and March 2004 in Leuven University Hospital Gasthuisberg. All autopsies were performed between 6 and 24 h after death. The presence of congenital malformations involving the gastrointestinal tract was considered as an exclusion criterion.
At autopsy, a careful in situ examination of the EGJ was carried out. A hiatus hernia was absent in all cases. About 1 cm of the distal esophagus and the whole stomach were excised together as a single piece, with a ring of diaphragmatic muscle. Two specimens were snap-frozen in isopentane, cooled by liquid nitrogen and stored at -80 °C. The other specimens were opened along the greater curvature or transversely and fixed overnight in 10% neutral-buffered formalin. The fixed specimens were routinely processed and embedded in paraffin.
Five-micrometer-thick step sections of all cases were stained with H&E and evaluated microscopically to select sections showing the EGJ mucosae. The number of sections selected varied between 10 and 40.
The epithelial lining of the EGJ was classified into five types according to Salenius[20] and Chandrasoma[21]. Foregut embryonic epithelium was defined as pseudostratified columnar epithelium consisting of undifferentiated cells; esophageal ciliated epithelium as multilayered epithelium covered by ciliated cells; esophageal squamous epithelium as non-keratinizing multilayered squamous epithelium; esophageal simple columnar epithelium without parietal cells (ESN) as simple columnar epithelium which is either flat or shows crypts, but is devoid of parietal cells; and esophageal simple columnar epithelium with parietal cells (ESP) as simple columnar epithelium with crypts and parietal cells.
The following parameters were measured at four evenly spaced points along the esophageal perimeter: length of abdominal esophagus (defined as the distance between the upper rim of the attachment of the diaphragm to the esophagus and the level of the angle of His); length of esophagus lined by simple columnar epithelium (defined as the distance from the squamocolumnar junction to the level of the angle of His); and length of ESN. These distances were measured along the mucosal surface using a calibrated graticule in the eyepiece of a Leitz DMRB microscope (objectives: Fluotar, eyepieces: L PLAN 10×/25).
Serial sections were immunostained with mAbs for CK5, 7, 8, 13, 18, 19, and 20. Murine mAbs against human CK7, 18, 19, and 20 were purchased from DakoCytomation (Glostrup, Denmark); mAbs against CK5, 8, and 13 were purchased from Novocastra Laboratories (Newcastle upon Tyne, UK, Table 1). After standard avidin-biotin-peroxidase complex immunostaining, all slides were counterstained with hematoxylin. Positive controls consisted of sections from prostate (CK5, 18, and 19), bile duct (CK7 and 8), tonsil (CK13), and ileum (CK20). Negative controls consisted of slides without application of primary antibody.
| Antigen | Clone | Antigen retrieval technique1 | Application of primary Ab2 |
| CK5 | XM26 | M | 1:50; 1 h; room temperature |
| CK7 | OV-TL 12/30 | T | 1:50; 1 h; room temperature |
| CK8 | TS1 | M | 1:50; 1 h; room temperature |
| CK13 | KS-1A3 | M | 1:50; 1 h; room temperature |
| CK18 | DC 10 | M | 1:10; 1 h; room temperature |
| CK19 | RCK108 | M | 1:20; 1 h; room temperature |
| CK20 | Ks20.8 | T | 1:50; 1 h; room temperature |
Antibody staining was graded for location, intensity (absent, weak, moderate, or strong) and degree (focal = <50% of epithelium, diffuse = ≥50% of epithelium) and compared with controls. The coordinate CK7/CK20 pattern was defined as CK7 positivity at least in surface and pit cells, and CK20 positivity at least in surface cells. The slides were scored independently by two of the authors (GDH and KG), and differences in interpretation were resolved by consensus at a multihead microscope.
The Pearson correlation coefficient r was calculated to investigate the degree of linear relationship between two continuous variables. A Fisher’s r to z transformation was carried out on the correlation allowing the calculation of a P-value for the null hypothesis that the correlation was equal to zero. The ANOVA test was used to study the effect of a nominal independent variable on a continuous dependent variable. To detect relationships between two nominal variables, the χ2 test or the Fisher’s exact test was used, wherever appropriate. P values <0.05 were considered significant.
Of the 48 autopsy specimens included in this study, 9 were rejected because of epithelial dehiscence, delocalization and fragmentation due to autolysis. The remaining 39 autopsy specimens belonged to 1 embryo of 7 wk gestational age (GA), 35 fetuses (GA: range 12-30 wk, mean±SD: 20±4 wk) and 3 neonates (of 38, 40, and 43 wk GA) (Table 1).
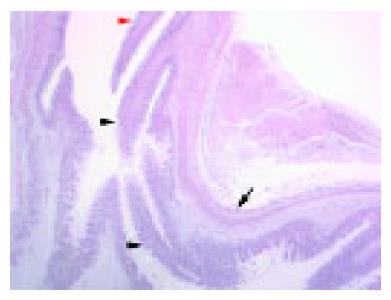
At 7 wk GA, both esophagus and stomach were lined by foregut embryonic epithelium, which contained depressions surrounded by radially placed cells along the lesser curvature of the stomach (Figure 1).
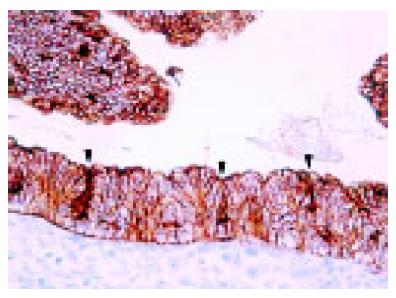
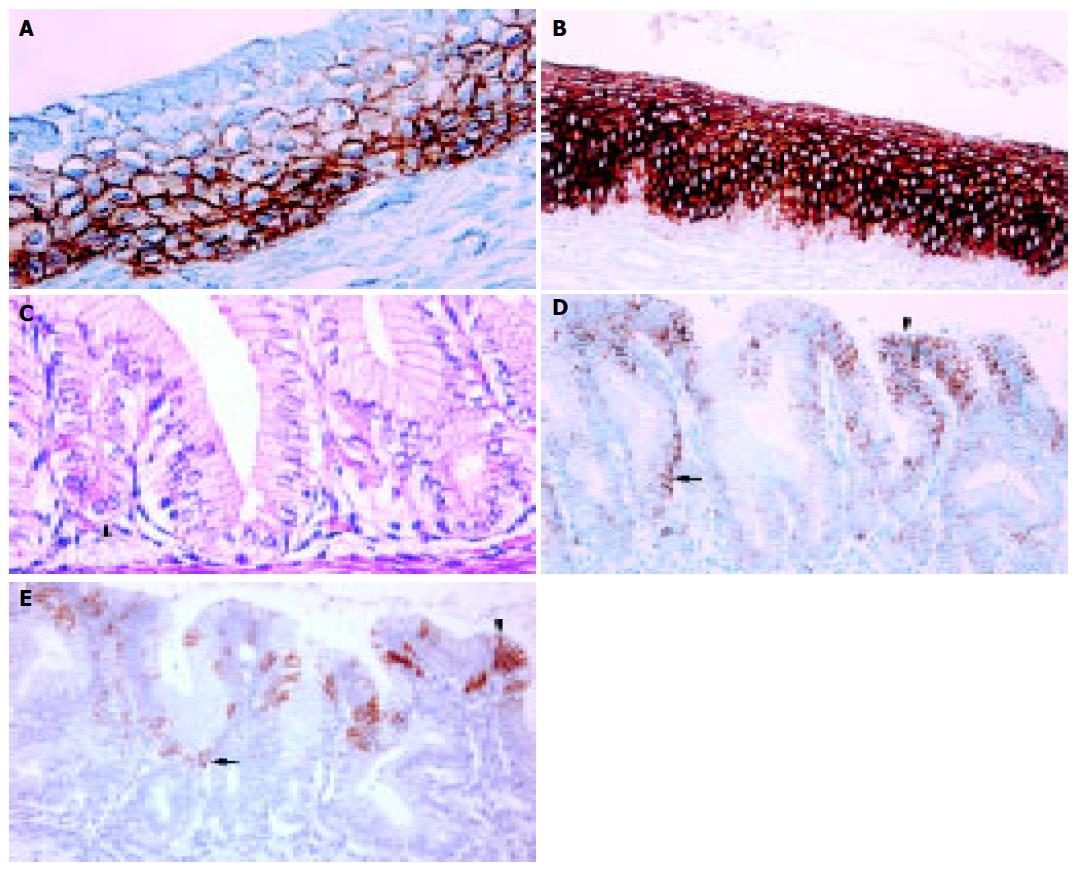
The distal end of the esophagus was located at the level of the angle of His. This anatomic boundary between the tubular esophagus and the saccular stomach was easily identifiable in the microscopy slides of all cases from 12-wk GA (Figure 2). At this time point, the tubular esophagus was lined by ciliated epithelium (Figure 3A), except in its most distal part. With advancing pregnancy, the ciliated epithelium was progressively replaced by squamous epithelium. Ciliated cells were absent from 40 wk GA (Figure 3B).
The distal esophagus was lined by simple columnar epithelium from 12-wk GA. This segment was between 0.480 and 7.505 mm in length (mean±SD: 2.185±1.619 mm). Its length increased significantly during gestation (r = 0.344, P = 0.0494), as did the length of the abdominal esophagus (r = 0.540, P = 0.0025). The length of esophageal simple columnar epithelium expressed as a percentage of length of the abdominal esophagus ranged between 21.569 and 100.000% (mean±SD: 50.884±18.259%). This percentage remained nearly constant during gestation (r = -0.140, P = 0.4815).
Throughout gestation, esophageal simple columnar epithelium could be divided into two parts (Figure 3C). The distal part contained parietal cells with roughly triangular shape and highly acidophilic cytoplasm (ESP). This segment was continuous with the stomach fundus. The proximal part of the esophageal simple columnar epithelium, however, was always devoid of parietal cells (ESN). Crypts were present over the entire length of the ESN from 16 wk GA. The length of the ESN ranged between 0.160 and 1.308 mm (mean±SD: 0.612±0.304 mm). There was no significant difference between the lengths of ESN at four evenly spaced points along the esophageal perimeter (ANOVA test, P = 0.4704). The length of ESN expressed as a percentage of total length of esophageal simple columnar epithelium ranged between 8.120% and 91.447% (mean±SD: 38.499±24.089%). This percentage decreased significantly during gestation (r = -0.572, P = 0.0004).
The CK immunostaining patterns for all locations are summarized in Table 2.
| Location | CK5 | CK7 | CK8 | CK13 | CK18 | CK19 | CK20 |
| Foregut embryonic epithelium (n = 1) | A | A | M (D) | A | M (D) | M (D) | A |
| Esophageal ciliated epithelium (n = 36) | |||||||
| Basal cell layer | S (D) | A | M (F) | A | A | M (D) | A |
| Columnar cell layer, non-ciliated cells | S (D) | M (D) | M (D) | S (D) | W (F) | M (D) | A |
| Columnar cell layer, ciliated cells | M (F) | M (D) | M (D) | M (D) | M (F) | M (D) | A |
| Esophageal squamous epithelium (n = 2) | |||||||
| Basal cell layer | S (D) | A | A | A | A | M (D) | A |
| Prickle cell layer | S (D) | W (F) | A | M (D) | A | M (D) | A |
| Superficial cell layer | M (D) | W (F) | A | S (D) | A | M (D) | A |
| ESN (n = 38) | |||||||
| Surface cells | A | M (F) | M (D) | A | M (D) | S (D) | A (1) |
| Pit cells | A | M (F) | M (D) | A | M (D) | M (D) | A |
| Deepest crypt cells | A | W (F) | M (D) | A | M (D) | M (D) | A |
| ESP (n = 38) | |||||||
| Surface cells | A | M (F) | M (D) | A | M (D) | S (D) | A (2) |
| Pit cells | A | M (F) | M (D) | A | S (D) | S (D) | A (3) |
| Parietal cells | A | A | M (D) | A | S (D) | M (D) | A |
| Stomach body mucosa (n = 38) | |||||||
| Surface cells | A | M (F) | M (D) | A | M (D) | M (D) | A (4) |
| Pit cells | A | M (F) | M (D) | A | M (D) | M (D) | A (5) |
| Glandular cells | A | A | M (D) | A | M (D) | M (D) | A |
| Stomach antrum mucosa (n = 10) | |||||||
| Surface cells | A | A | S (D) | A | M (D) | S (D) | S(F) |
| Pit cells | A | A | S (D) | A | M (D) | S (D) | M(F) |
| Glandular cells | A | A | M (D) | A | M (D) | M (D) | A |
CK5 and CK13 were first detected in a fetus of 14 wk GA. They were seen exclusively in multilayered epithelia. CK5 staining was stronger and more diffuse in the basal and intermediate cell layers than in the superficial cell layer (Figure 3A). CK13 staining was diffused in the intermediate and superficial cell layers (Figure 3B). Its staining intensity increased significantly throughout gestation (r = 0.476 and 0.523 respectively, P<0.0001).
CK8, CK18, and CK19 were all present in foregut embryonic epithelium (Figure 1). CK8 and CK18 staining were less intense in multilayered epithelia than in simple columnar epithelium. In ciliated epithelium, the basal cell layer was more often negative for these CKs than the other cell layers. In simple columnar epithelium, parietal cells stained weaker for CK8 and CK19 than mucus-secreting cells.
CK7 was first detected at 12-wk GA. Throughout gestation, it was more commonly present in the esophagus than in the stomach (Figure 3D). In multilayered epithelia, basal cells were always negative. Parietal cells contained no CK7, whereas the deepest crypt cells in the ESN were focally and weakly positive. This was the only statistically significant difference between the CK immunophenotypes of ESN and ESP (χ2: P<0.0001).
CK20 was seen in the stomach from 14 wk GA and in the esophagus from 17 wk GA (Figure 3E). It was expressed in the surface or pit cells of simple columnar epithelia. Expression of CK20 was always focal.
Coordinate CK7/20 expression for all locations is summarized in Table 3. We observed no statistically significant differences in the frequencies of the CK7+/20+ and the CK7-/20- immunophenotypes between different locations. The prevalence of the CK7+/20- immunophenotype decreased significantly from proximal to distal (33% in esophageal simple columnar epithelium vs 0% in the stomach, Fisher’s exact test P = 0.0039). The positive predictive value of the CK7+/20- immunophenotype for location in the esophagus (vs location in the stomach) was 100%. The usefulness of this feature was limited however, by its low sensitivity (33%). The prevalence of the CK7-/20+ immunophenotype increased significantly from proximal to distal (0% in ESN vs 40% in the stomach antrum, Fisher’s exact test P = 0.0285).
| Location | CK7+/20- (%) | CK7+/20+ (%) | CK7-/20+ (%) | CK7-/20- (%) |
| Fetal CLE (n = 24) | 8 (33) | 6 (25) | 2 (8) | 8 (33) |
| ESN (n = 22) | 8 (36) | 4 (18) | 0 (0) | 10 (45) |
| ESP (n = 22) | 4 (18) | 5 (23) | 3 (14) | 10 (45) |
| Stomach (n = 23) | 0 (0) | 4 (17) | 4 (17) | 15 (65) |
| Body mucosa (n = 23) | 0 (0) | 3 (13) | 5 (22) | 15 (65) |
| Antrum mucosa (n = 5) | 0 (0) | 1 (20) | 2 (40) | 2 (40) |
In this study, we examined the histology and CK expression patterns of the esophageal and gastric mucosae in fetuses and neonates, using autopsy material. We believe that the results of such a study may be relevant to the description of the normal adult situation. This is important in view of the current controversy on the origin and significance of CM. Our results can be summarized as follows: (1) the distal esophagus was lined by simple columnar epithelium from 12-wk GA; (2) the proximal part of this segment consisted of mucus-producing epithelium devoid of parietal cells; and (3) the reported adult CK expression pattern was established during gestation.
We found that the distal esophagus was lined by simple columnar epithelium from 12-wk GA. We propose the term “fetal columnar-lined lower esophagus s” (fetal CLE) for this condition. Fetal CLE has been mentioned only infrequently until now. Salenius states that “The gastroesophageal limit is by no means sudden, but the epithelial change occurs quite abruptly. Glands typical of the esophagus are found over a distance of a few millimeters in the cardiac area at the junction of the esophagus and stomach.”[20] Thus, it may be difficult to decide whether simple columnar epithelium is actually located in the distal esophagus or in the proximal stomach of fetuses. Ellison et al[22], mentioned the presence of “the squamocolumnar junction in the tubular esophagus proximal to the gastric dilatation” in 1 case out of 49 fetal and pediatric autopsies. They considered this finding suggestive of a hiatus hernia. We excluded the presence of hiatal hernia by a careful in situ examination of the EGJ at autopsy. In our material, fetal CLE had a length ranging between 0.480 and 7.505 mm, with a mean of 2.185 mm. We admit that these segments of simple columnar epithelium are very short. This might influence the decision whether to locate the simple columnar epithelium in the fetal esophagus or stomach. However, we are quite sure that this simple columnar epithelium was in the distal esophagus in our cases. We have two arguments: (1) the angle of His, the level of which marks the distal margin of the tubular esophagus, was always easily identifiable; and (2) the length of esophageal simple columnar epithelium expressed as a percentage of length of the abdominal esophagus ranged between 22% and 100%, with a mean of 51%. The last finding indicates that the fetal squamo-columnar junction was located definitely in the abdominal esophagus, often mid-way between the angle of His and the diaphragm.
The significance of fetal CLE is unclear. It is known since 1953 that the most distal part of the adult tubular esophagus can be lined by columnar epithelium[23]. Since the 1970 landmark experimental report by Bremner et al[24], it has been widely accepted that adult columnar-lined esophagus is an acquired condition in which the squamous esophageal epithelium destroyed by gastroesophageal reflux is replaced by columnar cells. However, even normal subjects may have short segments of columnar epithelium within 2 cm of the proximal margin of the gastric folds[25,26]. In our material, the length of fetal CLE showed no tendency to decrease during gestation. Moreover, its length expressed as a percentage of abdominal esophagus length remained nearly constant. The fetal squamo-columnar junction thus had an almost constant position. Therefore, the possibility exists that fetal CLE may be the equivalent (and the precursor) of the short segments of columnar epithelium found in the distal esophagus of some normal adult subjects. On the other hand, we cannot formally exclude that fetal CLE may differentiate into squamous epithelium in the esophagus and gastric mucosa in the stomach once the gastroesophageal sphincter is fully developed[21].
From 12-wk GA, the fetal CLE could be divided into two parts. Parietal cells were present in the distal part, which was continuous with the fundus part of the fetal stomach. We call this part of the fetal CLE as ESP. ESP may be the equivalent of oxyntocardiac mucosa, which was first described by Chandrasoma et al[7]. Oxyntocardiac mucosa differs from pure oxyntic mucosa by the absence of chief cells in the former.
The proximal part of the fetal CLE was lined circumf-erentially by a mucus-producing epithelium devoid of parietal cells. We call this part of the fetal CLE as ESN. From 16-wk GA, ESN contained crypts over its entire length at least as deep as those in the ESP. Therefore, we assume that mucus-secreting glandular structures devoid of parietal cells are present proximally in the fetal CLE. This description equates the histological definition of adult CM[4,7,8]. Conse-quently, we consider ESN as the equivalent (and forerunner) of adult CM. An alternative name for ESN would thus be 揻etal CM. Besides the histologic aspect of the epithelium, several other arguments are in favor of this conclusion. Like adult CM, ESN is located between multilayered esophageal epithelium and simple columnar epithelium with parietal cells. Also like adult CM, it is very short (range in our material: 0.1601.308 mm, mean 0.612 mm)[7,8]. Even its position in the distal esophagus cannot be objected, if one accepts that fetal CLE may be the precursor of the short segments of columnar epithelium found in the distal esophagus in some normal adults. Our view on ESN is shared by other investigators[14].
Since classical morphological techniques cannot definitely solve the riddle of the origin of adult CM, we also investigated the CK expression patterns of the developing EGJ and stomach. We chose to do this for two reasons: (1) there is a large body of literature on CK expression in the adult EGJ mucosae, particularly on the so-called Barrett’s CK7/20 pattern[15-18]; and (2) Barrett’s CK7/20 pattern has also been found in adult CM and this similarity to intestinal metaplastic epithelium in Barrett’s esophagus has been used as an argument for its metaplastic origin[17,18].
CK expression in the adult EGJ mucosae has been invest-igated by Glickman et al[27]. CK13 positivity was restricted to esophageal squamous epithelium. The basal layer of this epithelium stained for CK19 in 90% of cases. CK7 and CK8/18 were present focally and weakly in 61% and 18% of cases, respectively. CK7, 8/18, 19, and 20 were present in all biopsies of esophageal columnar and gastric cardiac epithelium. CK20 was only seen in columnar epithelia, where it was present in all cases. The CK expression pattern of adult ciliated respiratory epithelium has been studied by Hicks et al[28]. Basal cells typically stained for CK5, and columnar cells were positive for CK7, 8, and 18. CK19 was positive in both cell types. CK13 expression was not detected.
We observed similar CK expression patterns in the fetal EGJ mucosae. The only notable difference from the adult situation was the initially weak expression of CK13 in multilayered epithelia. However, CK13 immunostaining increased significantly throughout gestation. This finding may be related to the maturation of ciliated into squamous esophageal epithelium.
The co-ordinated CK7/20 immunophenotypes of the non-intestinalized mucosae of the adult EGJ and stomach have been described and illustrated in several publications[29-31]. From these data, it seems that in general CK7 positivity is more common and more extensive in CM than in gastric body or antrum mucosa. Conversely, CK20 positivity is more intense and more widespread in the stomach. These observations are concordant with our findings showing that the prevalence of the CK7+/20- immunophenotype decreases significantly from fetal CM to fetal antrum, while the prevalence of the CK7-/20+ immunophenotype increases significantly in the same direction.
The fetal CK7+/20+ immunophenotype in the esophagus is illustrated in Figures 3D and E, showing patchy superficial CK20 positivity and superficial and deep CK7 positivity. This pattern is similar to the expression reported in non-intestinalized CM by DeMeester et al[18]. The patchy superficial CK20 positivity is slightly different from the adult Barrett’s CK7/20 pattern. Ormsby et al[15,16], studied CK7/20 expression in LSBE. They described the Barrett’s CK7/20 pattern as band-like (i.e., diffuse) CK20 staining of surface epithelium and superficial glands, with moderate to strong CK7 staining of both superficial and deep glands in the specialized mucosa.
The fetal equivalent to adult CM thus shows already co-ordinated CK7/20 expression (in our material in 25% of cases). Together with the other similarities between the fetal and adult EGJ and stomach CK expression patterns, this supports the conclusion that a cardia-type mucosa is established during gestation. This would then indicate that at least a part of the adult CM has a congenital origin.
| 1. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 2. | Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510-513. [PubMed] |
| 3. | Spechler SJ. The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology. 1999;117:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Oberg S, Peters JH, DeMeester TR, Chandrasoma P, Hagen JA, Ireland AP, Ritter MP, Mason RJ, Crookes P, Bremner CG. Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg. 1997;226:522-530; discussion 530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 219] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Goldblum JR, Vicari JJ, Falk GW, Rice TW, Peek RM, Easley K, Richter JE. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Csendes A, Smok G, Christensen H, Rojas J, Burdiles P, Korn O. [Prevalence of cardial or fundic mucosa and Helicobacter pylori in the squamous-columnar mucosa in patients with chronic patological gastroesophageal reflux without intestinal metaplasia comparated with controls]. Rev Med Chil. 1999;127:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Chandrasoma PT, Lokuhetty DM, Demeester TR, Bremmer CG, Peters JH, Oberg S, Groshen S. Definition of histopathologic changes in gastroesophageal reflux disease. Am J Surg Pathol. 2000;24:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Chandrasoma PT, Der R, Ma Y, Dalton P, Taira M. Histology of the gastroesophageal junction: an autopsy study. Am J Surg Pathol. 2000;24:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 141] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Park YS, Park HJ, Kang GH, Kim CJ, Chi JG. Histology of gastroesophageal junction in fetal and pediatric autopsy. Arch Pathol Lab Med. 2003;127:451-455. [PubMed] |
| 10. | Ormsby AH, Kilgore SP, Goldblum JR, Richter JE, Rice TW, Gramlich TL. The location and frequency of intestinal metaplasia at the esophagogastric junction in 223 consecutive autopsies: implications for patient treatment and preventive strategies in Barrett's esophagus. Mod Pathol. 2000;13:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Kilgore SP, Ormsby AH, Gramlich TL, Rice TW, Richter JE, Falk GW, Goldblum JR. The gastric cardia: fact or fiction? Am J Gastroenterol. 2000;95:921-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Zhou H, Greco MA, Daum F, Kahn E. Origin of cardiac mucosa: ontogenic consideration. Pediatr Dev Pathol. 2001;4:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Glickman JN, Fox V, Antonioli DA, Wang HH, Odze RD. Morphology of the cardia and significance of carditis in pediatric patients. Am J Surg Pathol. 2002;26:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Derdoy JJ, Bergwerk A, Cohen H, Kline M, Monforte HL, Thomas DW. The gastric cardia: to be or not to be? Am J Surg Pathol. 2003;27:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Ormsby AH, Goldblum JR, Rice TW, Richter JE, Falk GW, Vaezi MF, Gramlich TL. Cytokeratin subsets can reliably distinguish Barrett's esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Ormsby AH, Vaezi MF, Richter JE, Goldblum JR, Rice TW, Falk GW, Gramlich TL. Cytokeratin immunoreactivity patterns in the diagnosis of short-segment Barrett's esophagus. Gastroenterology. 2000;119:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Glickman JN, Wang H, Das KM, Goyal RK, Spechler SJ, Antonioli D, Odze RD. Phenotype of Barrett's esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction: an immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45 MI. Am J Surg Pathol. 2001;25:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | DeMeester SR, Wickramasinghe KS, Lord RV, Friedman A, Balaji NS, Chandrasoma PT, Hagen JA, Peters JH, DeMeester TR. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett's esophagus. Am J Gastroenterol. 2002;97:2514-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | De Hertogh G, Van Eyken P, Ectors N, Tack J, Geboes K. On the existence and location of cardiac mucosa: an autopsy study in embryos, fetuses, and infants. Gut. 2003;52:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Salenius P. On the ontogenesis of the human gastric epithelial cells. A histologic and histochemical study. Acta Anat Suppl (Basel). 1962;50:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Chandrasoma PT. Fetal "cardiac mucosa" is not adult cardiac mucosa. Gut. 2003;52:1798; author reply 1798-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Ellison E, Hassall E, Dimmick JE. Mucin histochemistry of the developing gastroesophageal junction. Pediatr Pathol Lab Med. 1996;16:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax. 1953;8:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 278] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Bremner CG, Lynch VP, Ellis FH. Barrett's esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68:209-216. [PubMed] |
| 25. | McClave SA, Boyce HW, Gottfried MR. Early diagnosis of columnar-lined esophagus: a new endoscopic diagnostic criterion. Gastrointest Endosc. 1987;33:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 155] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Csendes A, Maluenda F, Braghetto I, Csendes P, Henriquez A, Quesada MS. Location of the lower oesophageal sphincter and the squamous columnar mucosal junction in 109 healthy controls and 778 patients with different degrees of endoscopic oesophagitis. Gut. 1993;34:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol. 2001;25:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Hicks W, Ward R, Edelstein D, Hall L, Albino A, Hard R, Asch B. Cytokeratin expression in human respiratory epithelium of nasal polyps and turbinates. Cell Biol Int. 1995;19:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Jovanovic I, Tzardi M, Mouzas IA, Micev M, Pesko P, Milosavljevic T, Zois M, Sganzos M, Delides G, Kanavaros P. Changing pattern of cytokeratin 7 and 20 expression from normal epithelium to intestinal metaplasia of the gastric mucosa and gastroesophageal junction. Histol Histopathol. 2002;17:445-454. [PubMed] |
| 30. | Mohammed IA, Streutker CJ, Riddell RH. Utilization of cytokeratins 7 and 20 does not differentiate between Barrett's esophagus and gastric cardiac intestinal metaplasia. Mod Pathol. 2002;15:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Flucke U, Steinborn E, Dries V, Mönig SP, Schneider PM, Thiele J, Hölscher AH, Dienes HP, Baldus SE. Immunoreactivity of cytokeratins (CK7, CK20) and mucin peptide core antigens (MUC1, MUC2, MUC5AC) in adenocarcinomas, normal and metaplastic tissues of the distal oesophagus, oesophago-gastric junction and proximal stomach. Histopathology. 2003;43:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK