Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4439
Revised: October 1, 2004
Accepted: October 4, 2004
Published online: July 28, 2005
AIM: To evaluate whether intratumoral injection of liposome-endostatin complexes could enhance the antitumor efficacy of radiation therapy in human liver carcinoma (BEL7402) model.
METHODS: Recombinant plasmid pcDNA3.End was transfected into human liver carcinoma cell line (BEL7402) with lipofectamine to produce conditioned medium. Then BEL7402 cells and human umbilical vein endothelial cells (HUVECs) were treated with the conditioned medium. Cell cycle and apoptosis were analyzed by flow cytometer and endothelial cell proliferation rates were determined by MTT assay. The antitumor efficacy of endostatin gene combined with ionizing radiation in mouse xenograft liver tumor was observed.
RESULTS: Endostatin significantly suppressed the S phase fraction and increased the apoptotic index in HUVECs. In contrast, endostatin treatment had no effect on BEL7402 cell apoptosis (2.1 ± 0.3% vs 8.9 ± 1.3%, t = 8.83, P = 0.009<0.01) or cell cycle distribution (17.2 ± 2.3% vs 9.8 ± 1.2%, t = 4.94, P = 0.016<0.05). The MTT assay showed that endostatin significantly inhibited the proliferation of HUVECs by 46.4%. The combination of local endostatin gene therapy with radiation therapy significantly inhibited the growth of human liver carcinoma BEL7402 xenografts, the inhibition rate of tumor size was 69.8% on d 28 compared to the untreated group. The tumor volume in the pcDNA3.End combined with radiation therapy group (249 ± 83 mm3) was significantly different from that in the untreated group (823 ± 148 mm3, t = 5.86, P = 0.009<0.01) or in the pcDNA3 group (717 ± 94 mm3, t = 6.46, P = 0.003<0.01). Endostatin or the radiation alone also inhibited the growth of liver tumor in vivo, but their inhibition effects were weaker than those of endostatin combined with radiation, the inhibition rates on d 28 were 44.7% and 40.1%, respectively.
CONCLUSION: Endostatin not only significantly suppresses tumor growth but also enhances the antitumor efficacy of radiation therapy in human carcinoma xenograft.
- Citation: Zheng AQ, Song XR, Yu JM, Wei L, Wang XW. Liposome transfected to plasmid-encoding endostatin gene combined with radiotherapy inhibits liver cancer growth in nude mice. World J Gastroenterol 2005; 11(28): 4439-4442
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4439
Tumors are dependent on angiogenesis for sustained growth[1]. Endostatin, an endogenous antiangiogenic agent, is a Mr 20 000 COOH-terminal fragment of collagen XVIII. It is a potent inhibitor of angiogenesis in vitro, and has significant antitumor effects in a variety of preclinical tumor models[2,3]. Endostatin specifically inhibits endothelial cell proliferation without direct effects on tumor cell or non-neoplastic cell growth[4-7], whose overexpression can lead to primary tumor regression and growth inhibition[8].
Most therapeutic investigations of endostatin utilized the purified protein, but the protein purification process is difficult and may denature endostatin. For maintaining therapeutically effective serum levels the protein must be repeatedly used because it has a short half-life in vivo. One possible approach to overcome this problem may be the utilization of gene therapy strategy. Studies using viral vectors to deliver endostatin gene have demonstrated its efficacy in treatment of mouse tumor models[9-11].
Although antiangiogenic therapies have shown significant antitumor effects in preclinical investigations, angiogenesis inhibitors cannot achieve tumor cures on their own. Antiang-iogenic strategies in combination with conventional ant-icancer approaches may achieve better results. The invol-vement of antiangiogenic agents during the course of radiotherapy have been shown to produce significant therapeutic effects[12-15].
In this study, we investigated whether intratumoral injection of liposome-endostatin complexes could enhance the treatment efficacy of ionizing radiation in a human liver carcinoma (BEL7402) model.
The plasmid pcDNA3.End containing a synthetic rat insulin leader sequence and the full-length mouse endostatin cDNA was kindly provided by Dr. Wang Jianli (Shandong Medical University, Jinan, China). The synthetic rat insulin leader was cloned in front of the endostatin gene. Human liver carcinoma cell line BEL7402 and human umbilical vein endothelial cell line (HUVEC) were kept in our laboratory. HUVECs and BEL7402 cells were maintained in DMEM (Gibco) containing 10% FBS.
BEL7402 cells were grown in 6-well plates at the density of 2×105 cells/well to 50-80% confluence, then transfected with 10 mL of lipofectamine (Invitrogen) mixed with 4 mg of plasmid (pcDNA3.End or pcDNA3) as described by the Invitrogen protocol. After 24 h transfection, the cells were extensively rinsed with PBS and incubated in serum-free DMEM for another 24 h. The conditioned media were collected, centrifuged and cell debris was cleared off. Endostatin in the culture media was measured with a murine endostatin enzyme immunoassay kit (Chemicon Inc.). The conditioned media were concentrated 20-fold with Amicon membranes (Amicon Inc.), and endostatin protein levels were determined by immunoassay before being stored at -80 °C for further use.
BEL7402 cells and HUVECs were plated in 6-well plates at the density of 2×105 cells/well and allowed to attach overnight. The cells were treated with conditioned medium containing certain concentration of endostatin and 10% FBS after removal of the medium. Forty-eight hours later, the cells were trypsinized, counted and fixed in 50% ethanol overnight, then treated with PBS (containing 1 g/L RNase) for 30 min. Samples were washed with PBS twice and resuspended in PBS at a concentration of 1×106 cells/mL. The cells were stained with PI in darkness for 30 min and cell cycle distribution was analyzed with a flow cytometer (Becton-Dickinson FACS Calibur).
BEL7402 cells and HUVECs were cultured in 6-well plates at the density of 2×105 cells/well and incubated for 24 h. The medium was replaced with 2 mL of conditioned medium containing certain concentration of endostatin and 10% FBS. After being incubated for 48 h, the cells were trypsinized, counted, washed twice with cold PBS and then resuspended in 1 binding buffer at a concentration of 1×106 cells/mL. Five microliters of annexin V-FITC and five microliters of PI were added. The cells were gently vortexed and incubated for 15 min at RT in the dark, and then 400 mL of 1 binding buffer was added. Apoptosis was analyzed with a flow cytometer.
HUVECs were incubated in 96-well dishes at the density of 104 cells/well and allowed to attach overnight. The medium was then replaced with 20 mL of conditioned medium and incubated for 30 min. Eighty microliters of DMEM containing with 10% FBS and 1mg/L bFGF (Sigma) were then added. After the cells were incubated for 48 h, 20 mL MTT solution (5 g/L) was added. Then, 4 h later, 100 mL 100 g/L SDS was added. After being vortexed gently for 10 min, the number of cells was quantified by colorimetric MTT assay.
Female nude mice aged 4-6 wk were obtained from Animal Center of Shandong Medical University and fed with a standard rodent diet. To establish xenografts, animals were subcutaneously injected into the right flank with 1×106 BEL7402 cells suspended in 200mL 0.9% saline. Seven days after the injection of tumor cells, the mice were randomly divided into five treatment groups: untreated, empty vector (Lip-pcDNA3), Lip-pcDNA3.End, pcDNA3.End combined with radiation, and radiation. Six mice were enrolled in each group. To deliver the gene therapy, each mouse in groups 2, 3, and 4 received three intratumoral injections of liposome-DNA complex which consisted of 40 mL lipofectamine and 20 mg of DNA (1 g/L) and 40 mL 0.9% saline on d 7, 14, and 21 after the injection of tumor cells. Irradiations were performed using a 6 MV Varian 2100 C linear accelerator, operating a single dose of 10 Gy 7 d after injection of tumor cells. Before irradiation, the mice were confined in plastic containers. The animals tumor-bearing sites extended through openings in the containers allowing the tumors to be irradiated locally. Tumors were measured every 3-4 d. Tumor response to treatment was determined by growth delay assay. The tumor volume was calculated by the formula: tumor volume = ab20.52, where a is the length, and b is the width.
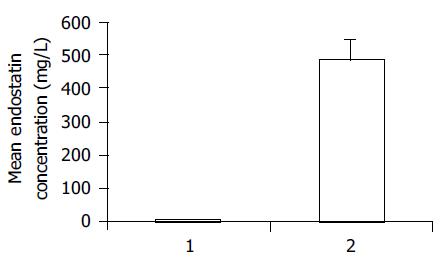
Unconcentrated media (100 mL) collected from BEL7402 cells transfected with pcDNA3.End and pcDNA3 were measured with a murine endostatin enzyme immunoassay kit. The experiment showed that BEL7402 cells transfected with pcDNA3.End efficiently secreted endostatin protein into the culture media. Endostatin levels were 486.2 ± 56.5 mg/L in conditioned media from BEL7402 cells transfected with pcDNA3.End, and 6.8 ± 2.6 mg/L in conditioned media from BEL7402 cells transfected with pcDNA3. There were significant differences in endostatin levels between the two groups (t = 14.68, P = 0.005<0.01, Figure 1).
After being treated with conditioned medium, compared to conditioned medium from BEL7402 cells transfected with pcDNA3, conditioned medium from BEL7402 cells transfected with pcDNA3.End significantly suppressed the S phase fraction (17.2 ± 2.3% vs9.8 ± 1.2%, t = 4.94, P = 0.016<0.05) and increased the apoptotic index (2.1 ± 0.3% vs 8.9 ± 1.3%, t = 8.83, P = 0.009<0.01) in HUVECs. In contrast, after being treated with the two-conditioned media respectively, there were no differences in BEL7402 cell apoptosis or cell cycle distribution, suggesting that endostatin treatment had no effect on BEL7402 tumor cell apoptosis or cell cycle distribution.
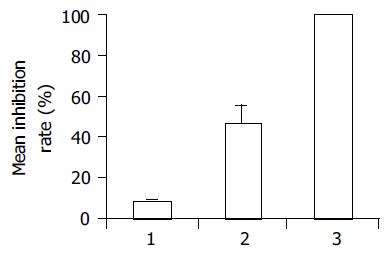
After 24 h incubation with conditioned media, endostatininhibited HUVECs proliferation by 46.4 ± 9.7%, while the conditioned media derived from cultures of BEL7402 cells transfected with pcDNA3 control vector did not affect endothelial cell proliferation, the inhibition rate was 8.7 ± 0.5% (t = 6.72, P = 0.02<0.05, Figure 2).
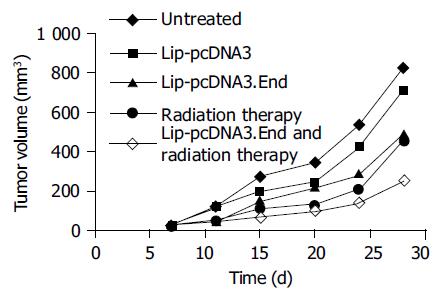
As shown in Figure 3, tumors treated with Lip-pcDNA3. End combined with radiation group grew very slowly in nude mice, the inhibition rate of tumor size was 69.8% on d 28 compared to untreated group. The tumor volume of the pcDNA3.End combined with radiation group (249 ± 83 mm3) was significantly different from that of the untreated group (823 ± 148 mm3, t = 5.86, P = 0.009<0.01) or the pcDNA3 group (717 ± 94 mm3, t = 6.46, P = 0.003<0.01). Tumors of the pcDNA3.End group and radiation group also grew slower than those of the untreated group or the pcDNA3 group, but the inhibitory effects on tumor growth were slightly weaker than those of the pcDNA3.
End combined with radiation group, the inhibitory rates on d 28 were 44.7% and 40.1%, respectively. The tumor volume of the pcDNA3.End group (492 ± 97 mm3) or the radiation group (455 ± 124 mm3) was significantly different from that of the untreated group (the pcDNA3.End group, t = 3.14, P= 0.039<0.05 and the radiation group, t = 3.30, P = 0.03<0.05) or the pcDNA3 group (the pcDNA3.End group, t = 2.89, P = 0.045<0.05 and the radiation group, t = 2.92, P = 0.047<0.05, Figure 3).
Radiotherapy is one of the most important treatment modalities for solid tumors. Today, 45-50% of all cancer patients can be cured, and nearly 70% of those who are cured have received radiation either alone or in combination with other modalities, but a large number of patients have no response to radiotherapy treated with curative intent ultimately fail, not only because of metastasis of the disease, but also because of relapse at the local treatment site. One reason responsible for radiotherapy failure may be the tumor vasculature. Numerous studies have shown that tumor cells stop growing when the diameter of tumor exceeds 1-2 mm if new blood vessels supplying the tumor fail to generate[16]. Hence, the combined radiotherapy with antiangiogenic agents has aroused great concerns[17,18].
Angiogenesis is a complex process with multiple, sequential, and interdependent steps. Tumor cells promote new vessel formation by releasing endothelial cell growth factors that support endothelial cell proliferation, migration, and survival. Tumor angiogenesis is the consequence of enhanced expression of proangiogenic factors relative to antiangiogenic factors in the tumor microenvironment.
The combination of radiation treatment with endostatin may improve radiotherapy outcome by enhancing antitumor efficacy, reducing the total radiation dose, improving local tumor control rate, alleviating radiation damage. These considerations, along with the extensive clinical use of radiotherapy, make thorough investigation of strategies combining conventional treatment modality with endostatin. It was reported that combined endostatin gene therapy with radiotherapy can improve tumor response[19-22].
We chose liposome to transfect BEL7402 cells with endostatin gene due to its high transfection efficiency. In our pre-experiment, we chose lipofectamine to transfect BEL7402 cells with pcDNA3.GFP, the transfection rate was 73.5%. ELISA analysis of conditioned media from BEL7402 cells transfected with pcDNA3.End showed that the level of endostatin protein was 486.2 ± 56.5 mg/L, suggesting that BEL7402 cells transfected with pcDNA3.End plasmids secrete endostatin proteins into the culture media. The conditioned medium significantly suppressed the S phase fraction and increased the apoptotic index in HUVECs. However, it had no effect on BEL7402 cell apoptosis or cell cycle distribution. In vivo, endostatin significantly enhanced the treatment efficacy of ionizing radiation. The antitumor inhibition rate of combined endostatin gene therapy with radiation in BEL7402 human liver tumor model was 69.8%, which was significantly different from the untreated group (t = 5.86, P = 0.009<0.01) or the empty vector group (t = 6.46, P = 0.003<0.01) on d 28. Tumors of the radiation group and the pcDNA3.End group grew slower than those of the untreated group or the pcDNA3 group. These results indicate that intratumoral injection of liposome-endostatin complex significantly enhances the antitumor efficacy of radiation therapy.
In summary, gene therapy can deliver antiangiogenic polypeptide endostatin. Cationic liposomes transfected to endostatin gene can not only suppress endothelial cell proliferation, but also enhance the treatment efficacy of ionizing radiation.
| 1. | Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5621] [Cited by in RCA: 5545] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 2. | Perletti G, Concari P, Giardini R, Marras E, Piccinini F, Folkman J, Chen L. Antitumor activity of endostatin against carcinogen-induced rat primary mammary tumors. Cancer Res. 2000;60:1793-1796. [PubMed] |
| 3. | Chen QR, Kumar D, Stass SA, Mixson AJ. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 1999;59:3308-3312. [PubMed] |
| 4. | Du Z, Hou S. The anti-angiogenic activity of human endostatin inhibits bladder cancer growth and its mechanism. J Urol. 2003;170:2000-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Wang X, Liu F, Li X, Li J, Xu G. Anti-tumor effect of human endostatin mediated by retroviral gene transfer in nude mice. Chin Med J (Engl). 2002;115:1664-1669. [PubMed] |
| 6. | Boehle AS, Kurdow R, Schulze M, Kliche U, Sipos B, Soondrum K, Ebrahimnejad A, Dohrmann P, Kalthoff H, Henne-Bruns D. Human endostatin inhibits growth of human non-small-cell lung cancer in a murine xenotransplant model. Int J Cancer. 2001;94:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Felbor U, Dreier L, Bryant RA, Ploegh HL, Olsen BR, Mothes W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000;19:1187-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 340] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Herbst RS, Lee AT, Tran HT, Abbruzzese JL. Clinical studies of angiogenesis inhibitors: the University of Texas MD Anderson Center Trial of Human Endostatin. Curr Oncol Rep. 2001;3:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Sauter BV, Martinet O, Zhang WJ, Mandeli J, Woo SL. Adenovirus-mediated gene transfer of endostatin in vivo results in high level of transgene expression and inhibition of tumor growth and metastases. Proc Natl Acad Sci USA. 2000;97:4802-4807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Ding XQ, Chen Y, Li L, Liu RY, Huang JL, Lai K, Wu XJ, Ke ML, Huang WL. Inhibition of tongue cancer development in nude mice transfected with adenovirus carrying human endostatin gene. Ai Zheng. 2003;22:1152-1157. [PubMed] |
| 11. | Chen W, Fu J, Liu Q, Ruan C, Xiao S. Retroviral endostatin gene transfer inhibits human colon cancer cell growth in vivo. Chin Med J (Engl). 2003;116:1582-1584. [PubMed] |
| 12. | Gorski DH, Mauceri HJ, Salloum RM, Gately S, Hellman S, Beckett MA, Sukhatme VP, Soff GA, Kufe DW, Weichselbaum RR. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res. 1998;58:5686-5689. [PubMed] |
| 13. | Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957-1971. [PubMed] |
| 14. | Griscelli F, Li H, Cheong C, Opolon P, Bennaceur-Griscelli A, Vassal G, Soria J, Soria C, Lu H, Perricaudet M. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc Natl Acad Sci USA. 2000;97:6698-6703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Harari PM, Huang SM. Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys. 2001;49:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770-4775. [PubMed] |
| 17. | Rofstad EK, Henriksen K, Galappathi K, Mathiesen B. Antiangiogenic treatment with thrombospondin-1 enhances primary tumor radiation response and prevents growth of dormant pulmonary micrometastases after curative radiation therapy in human melanoma xenografts. Cancer Res. 2003;63:4055-4061. [PubMed] |
| 18. | Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res. 2000;6:971-978. [PubMed] |
| 19. | Siemann DW, Shi W. Targeting the tumor blood vessel network to enhance the efficacy of radiation therapy. Semin Radiat Oncol. 2003;13:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Herbst RS, O'Reilly MS. The rationale and potential of combining novel biologic therapies with radiotherapy: focus on non-small cell lung cancer. Semin Oncol. 2003;30:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Shi W, Teschendorf C, Muzyczka N, Siemann DW. Gene therapy delivery of endostatin enhances the treatment efficacy of radiation. Radiother Oncol. 2003;66:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Science Editor Wang XL Language Editor Elsevier HK