Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4423
Revised: December 11, 2004
Accepted: December 14, 2004
Published online: July 28, 2005
AIM: To elucidate the mechanism of liver protection by inhibition of Kupffer cells (KCs) function.
METHODS: All the animals were randomly divided into three groups. Blockade group (gadolinium chloride solution (GdCl3) injection plus ischemia/reperfusion (I/R) injury): GdCl3 solution was injected once every 24 h for 2 d via the tail vein before I/R injury. Non-blockade group (saline solution injection plus I/R injury): saline instead of GdCl3 as a control was injected as in the blockade group. Sham group: saline was injected without I/R injury. Liver samples were collected 4 h after blood inflow restoration. The blockade of the function of KCs was verified by immunostaining with an anti-CD68 mAb. Toll-like receptor 2 (TLR2) was immunostained with a goat antimouse polyclonal anti-TLR2 antibody. Membrane proteins were extracted from the liver samples and TLR2 protein was analyzed by Western blot. Portal vein serum and plasma were taken respectively at the same time point for further detection of the levels of tumor necrosis factor-a (TNF-a) and alanine aminotransferase (ALT), an indicator of liver function.
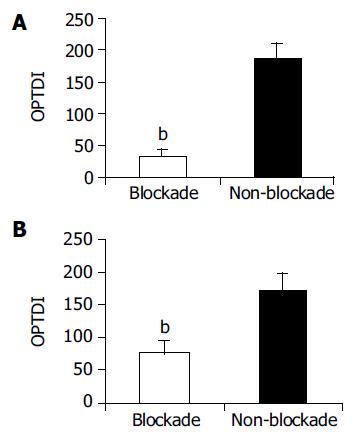
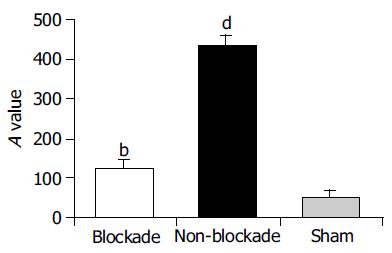
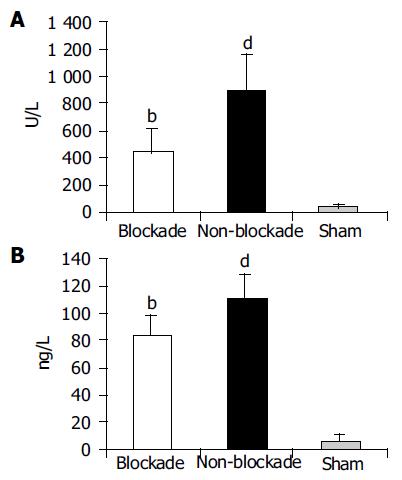
RESULTS: Compared to non-blockade group, CD68+ cells significantly reduced in blockade group (OPTDI, optical density integral): 32.97±10.55 vs 185.65±21.88, P<0.01) and the liver function impairment was relieved partially (level of ALT: 435.89±178.37 U/L vs 890.21±272.91 U/L, P<0.01). The expression of TLR2 protein in blockade group significantly decreased compared to that in non-blockade group (method of immunohistochemistry, OPDTI: 75.74±17.44 vs 170.58±25.14, P<0.01; method of Western blot, A value: 125.89±15.49 vs 433.91±35.53, P<0.01). The latter correlated with the variation of CD68 staining (r = 0.745, P<0.05). Also the level of portal vein TNF-a decreased in blockade group compared to that in non-blockade group (84.45±14.73 ng/L vs 112.32±17.56 ng/L, P<0.05), but was still higher than that in sham group (84.45±14.73 ng/L vs6.07±5.33 ng/L, P<0.01).
CONCLUSION: Inhibition of the function of KCs may protect liver against I/R injury via downregulation of the expression of TLR2.
- Citation: Zhang JX, Wu HS, Wang H, Zhang JH, Wang Y, Zheng QC. Protection against hepatic ischemia/reperfusion injury via downregulation of toll-like receptor 2 expression by inhibition of Kupffer cell function. World J Gastroenterol 2005; 11(28): 4423-4426
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4423.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4423
Hepatic ischemia/reperfusion (I/R) injury is one of the major complications of liver resection surgery, transplantation, and hypovolemic shock[1,2]. Although the detailed biochemical mechanisms are unclear, activation of Kupffer cells (KCs) may play an important role. During the very early phase of I/R injury, the secretion of proinflammatory cytokines by activated KCs, such as TNF-a, interleukin-1, participates in liver I/R injury, which precedes the activation of adhesion factors, chemotactic agents, and the sequestration of neutrophils in liver. Neutrophil accumulation in the liver causes direct hepatocellular damage through exhaustion of hepatic microcirculation by blocking the capillary perfusion and releasing proteases. So that we can see that KCs are among the first and key cells that mediate hepatic I/R injury[3]. Inhibition of the function of KCs elicits the protection against liver I/R injury. The mechanism of KCs in such an insult remains unclear. Endotoxin or lipopolysaccharide is a strong stimulator inducing KCs to secret proinflammatory mediators and ultimately leading to endotoxin-induced liver injury[4,5]. In order to clarify the mechanism of I/R injury without the effect of endotoxin and the corresponding cytokines evoked by endotoxin[5,6], we reproduced a lobar rather than total hepatic I/R injury in a mouse model to produce a severe hepatic ischemic insult without mesenteric venous congestion. So that the development of intestinal congestion and leakage of bacteria or bacterial products into the circulation can be avoided[6,7].
TLR family members, a kind of newly found transme-mbrane peptides, recognize pathogen-associated molecular patterns derived from both Gram-negative and Gram-positive bacteria such as endotoxin and are capable of sensitizing danger signals in in vivo environment[1,7,8]. TLRs participate in the initiation of the downstream inflammatory cascades[8,9]. In previous studies, we have proved that TLR2 and TLR4 are involved in the hepatic I/R pathologic process and the activation of them is not related to endotoxin[10,11]. Whether TLR2 expression is affected by the function of KCs is still unknown. GdCl3 is a kind of experimental drug, which is capable of blocking KC function specifically, while it has no effect on other macrophages, such as those residing in lung or intestinal canals[12,13]. CD68 is a pan-macrophage endosomal glycoprotein, which belongs to a family of acidic, highly glycosylated lysosomal glycoproteins and is found in cytoplasmic granules. It is considered as a specific indicator of KC activation[14]. That is why we used CD68 immunohistological staining to assess the inhibition of KC function.
This experiment aimed to observe the variation of TLR2 expression after the inhibition of the function of KCs and to further clarify the protective mechanism against hepatic I/R injury induced by inhibition of the function of KCs.
Male BALB/c mice weighing 20-25 g were supplied by Experimental Animal Center in Tongji Medical College. Their age ranged 6-8 wk. The animals were fasted for 12 h with free access to water and randomly divided into GdCl3 injection plus I/R injury group (blockade group), saline solution injection plus I/R injury group (non-blockade group), and sham operation group (sham group). The animals in blockade group received injections of GdCl3 solution (0.1 mmol/kg body weight, Sigma, USA) via tail vein once every 24 h for two times. The operation was performed 24 h after the last injection. The animals in non-blockade group were injected saline solution as control. Mice were anesthetized with pentobarbital (60 mg/kg). Laparotomy was performed through a midline incision and an atraumatic clip was placed across the hepatic hilar to interrupt blood supply to the left and median lobes of the liver. After 60 min of partial hepatic ischemia, the clip was removed to initiate hepatic reperfusion. Sham group mice underwent the same protocol as the control group without vascular occlusion. After the tissue was removed, animals were euthanized by injection of an overdose of pentobarbital. All studies were approved by the Institutional Animal Care and Use Committee of Tongji Medical College.
Liver samples from ischemic lobes were taken after 4 h of blood supply restoration and immediately fixed with 40 g/L formaldehyde, dehydrated, and embedded in paraffin for immu-nohistopathologic examination. Four micrometers of thick sections were stained with anti-CD68 Ab (Boster Bio. Co., Wuhan, China). The results were analyzed with a HPIAS pathological image analyzer and expressed as optical density integral (OPTDI).
Membrane proteins of ischemic liver tissues (100 mg) were extracted (1× PBS, 10 mL/L NP40, 5 g/L sodium desoxycho-late, 1 g/L sodium dodecyl sulfate, 10 g/L phenylmethylsulfonyl fluoride, 30 mL/L aprotinin, 1 mol/L sodium orthovanadate). After quantification and aliquot, the samples were degenerated by boiling, separated on 85 g/L SDS-PAGE and transferred to nitrocellulose membranes. Filters were blocked 50 g/L nonfat milk in blocking buffer (TBS-T, 50 mmol/L Tris-Cl, pH 7.5, 150 mmol/L NaCl, 0.2 g/L Tween 20), and incubated with anti-TLR2 antibody (Santa Cruz, CA, USA) for 2 h and with peroxidase-conjugated secondary antibody for 1 h at 37 °C. Specific bands were revealed with DAB solution and analyzed by Gel-Pro-Analyzer 4 as the value of A.
Other 4-mm-thick sections were stained with a goat antimouse TLR2 polyclonal antibody (Santa Cruz, CA, USA). The results were expressed as OPTDI and analyzed with a HPIAS pathological image analyzer.
The relationship between the expressions of CD68 and TLR2 was analyzed with Spearman, bivariate assay.
Blood samples from portal vein were taken for assay of plasma alanine aminotransferase (ALT) and TNF-a level. The ALT activity was determined by an automatic biochemistry analyzer (HITACHI 2000, Japan) in Laboratory of Union Hospital. TNF-a level was assayed by an ELISA kit (JINGMEI Bio. Co., China).
All numeric data were expressed as mean±SD. Differences between blockade and non-blockade groups were analyzed by t-test with SPSS 10.0 software. The correlation between CD68 staining and TLR2 expression was analyzed with Spearman assay. P<0.05 was considered statistically significant.
When KCs were blocked with GdCl3, the CD68+ reaction was weaker in blockade group than in non-blockade group (Figure 1A). CD68 content was shown as absolute value OPTDI (Area×OPTDM), which was 32.97±10.55 vs 185.65±21.88. The difference between them was significant (P<0.01), suggesting that the function of KCs was inhibited in blockade group.
The positive immunoreaction of TLR2 in slices was weaker in blockade group than in non-blockade group (Figure 1B), which was shown as OPDTI (75.74±17.44 vs 170.5825.14). The difference between them was significant (P<0.01).
After 4 h restoration of blood supply, the expression of TLR2 protein, which was detected by Western blot increased in the non-blockade group compared to sham group (A: 433.91±35.53 vs 52.86±13.58, P<0.01). The injection of GdCl3 significantly down regulated the expression of TLR2 in ischemic lobes compared to the non-blockade group (125.89±15.49 vs 433.91±35.53,P<0.01, Figure 2). Correlation analysis indicated that the downregulation of TLR2 expression between blockade and non-blockade groups was correlated with that of CD68 (r = 0.745, P<0.05).
After 4 h restoration of blood supply, the level of ALT in portal vein, an indicator of liver function, was higher in non-blockade group than in sham group (890.21±272.91 U/L vs 40.66±15.42 U/L, P<0.01).The value in blockade group decreased to 435.89±178.37 U/L. The difference between blockade and non-blockade groups was significant (P<0.01, Figure 3A).
The serum TNF-a level in portal vein was higher in non-blockade group than in sham group (112.32±17.56 ng/L vs 6.07±5.33 ng/L, P<0.01) after 4 h blood supply restoration. When the function of KCs was blocked by injection of GdCl3, the level of portal vein TNF-a was decreased remarkably (84.45±14.73 ng/L vs 112.32±17.56 ng/L, P<0.01), which might be an indirect indicator of KC inhibition (Figure 3B).
KCs play an important part in mediating ischemia and reperfusion injury[15]. When activated during ischemia and subsequent reperfusion, they generate excessive inflammatory cytokines and oxygen-derived free radicals, which play a particularly important role in the pathogenesis of hepatic ischemia and reperfusion injury with an increased release of TNF-a and histological impairment. GdCl3, a specific inhibitor of KCs, is often used as a tool for studying the role of KCs[16]. GdCl3 is capable of protecting liver from injury mediated by the activation of KCs through depletion of lipid peroxidation. In the present study, injection of GdCl3 selectively inhibited the activation of KCs, but did not induce hepatotoxicity. On the contrary, the impairment of liver function was relieved.
TLR2 can be activated during the process of hepatic I/R injury[10]. When TLR2 is activated, its cytoplasmic portion would conduct signals to two distinct signaling pathways, JNK and NF-kB, resulting in the proinflammatory cascade and excessive production of TNF-a[17]. TLRs recognize two kinds of signals. One is related to pathogen-associated molecular pattern molecules, which claims the existence of pathogens[9]; the other is linked with some danger signals inside or outside the body, which does not need the existence of pathogens[18]. Hypothesis infers that TLRs may be the candidate of a key "gate" through which the down stream inflammatory cascade is initiated[17]. TLR2 and TLR4 are activated in liver I/R injury[10,11], but the factors regulating the expression of TLRs have not been revealed.
Previous study has proved that NF-kB activation may be important in "switching off" the cytokine cascade during I/R injury. TNF-a plays a central role in mediating such an insult[7]. Nevertheless, what happens preceding the NF-kB activation and TNF-arelease? Where is the switch point that starts a crucial inflammatory cascade involving the activation of NF-kB during the I/R pathological process? Activation of TLR2 results in the activation of NF-kB[17], and NF-kB activation in the liver could be downregulated by KC blockade. In this paper, we confirmed that blocking the function of KCs downregulated the expression of TLR2 in ischemic lobes in mice partial hepatic I/R model, and the levels of TNF-a and ALT in portal vein decreased at the same time, suggesting that inhibition of KC function may protect liver from I/R injury by suppressing the release of TNF-a through downregulation of TLR2 expression. On the other hand, KCs might play an important role in mediating liver injuries and inflammatory disorders in the liver by changing the TLR2 signal transduction pathway. But compared to the sham group, the levels of portal vein TNF-a and ALT were still higher in blockade group, indicating that besides activation of KCs there are other factors regulating the expression of TLR2. Whether KCs themselves or cytokines secreted by KCs regulate the expression of TLR2 in ischemic lobes remains unclear.
KCs are major contributors to cytokine production in hepatic I/R injury[15]. Although many factors, including reactive oxygen species, Ca++ overload, adhesive molecules, nitrogen monoxide, etc, contribute to the pathogenesis of hepatic I/R injury, all these factors exert their role after the activation of KCs and the resulting inflammatory cascade[3]. Further study on inflammatory reaction controlled by TLRs and KCs may resolve the enigma of hepatic I/R injury.
Authors thank Professor Du-Jun Ye, Pathophysiology Department, Tongji Medical College, Huazhong University of Science and Technology, for his helpful discussions. An excellent technical assistance of Dr. Yun Yang is acknowledged.
| 1. | Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S-54S. [PubMed] |
| 5. | Lukkari TA, Järveläinen HA, Oinonen T, Kettunen E, Lindros KO. Short-term ethanol exposure increases the expression of Kupffer cell CD14 receptor and lipopolysaccharide binding protein in rat liver. Alcohol Alcohol. 1999;34:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Kojima Y, Suzuki S, Tsuchiya Y, Konno H, Baba S, Nakamura S. Regulation of pro-inflammatory and anti-inflammatory cytokine responses by Kupffer cells in endotoxin-enhanced reperfusion injury after total hepatic ischemia. Transpl Int. 2003;16:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 643] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197-5203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 353] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 10. | Zhang JX, Wu HS, Wang L, Zhang JH, Wang H, Zheng QC. TLR2 mRNA upregulation in ischemic lobes in mouse partial hepatic ischemia/reperfusion injury model. J Hust. 2004;24:144-146. |
| 11. | Wu HS, Zhang JX, Wang L, Tian Y, Wang H, Rotstein O. Toll-like receptor 4 involvement in hepatic ischemia/reperfusion injury in mice. Hepatobiliary Pancreat Dis Int. 2004;3:250-253. [PubMed] |
| 12. | Lee CM, Yeoh GC, Olynyk JK. Differential effects of gadolinium chloride on Kupffer cells in vivo and in vitro. Int J Biochem Cell Biol. 2004;36:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Gloor B, Todd KE, Lane JS, Lewis MP, Reber HA. Hepatic Kupffer cell blockade reduces mortality of acute hemorrhagic pancreatitis in mice. J Gastrointest Surg. 1998;2:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1996;93:14833-14838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 299] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Schümann J, Wolf D, Pahl A, Brune K, Papadopoulos T, van Rooijen N, Tiegs G. Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol. 2000;157:1671-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 234] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200-G207. [PubMed] |
| 17. | Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 937] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 18. | Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3013] [Cited by in RCA: 2733] [Article Influence: 113.9] [Reference Citation Analysis (11)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Co-first-authors: Jin-Xiang Zhang, He-Shui Wu and Hui Wang
Co-correspondence: He-Shui Wu