Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4404
Revised: November 13, 2004
Accepted: November 19, 2004
Published online: July 28, 2005
AIM: To investigate the possible role of c-erbB-2 and glutathione S-transferase (GST-Pi) in primary hepatocellular carcinogenesis and the relationship between liver hype-rplastic nodule (LHN), liver cirrhosis (LC), and hepatocellular carcinoma (HCC).
METHODS: The expression of c-erbB-2 and GST-Pi was detected immunohistochemically in 41 tissue specimens of HCC and 77 specimens of its adjacent tissue.
RESULTS: The positive expression of c-erbB-2 in LHN (28.6%) was significantly higher than that in LC (0%) (P = 0.032<0.05), but no significant difference was seen between HCC and LHN or LC (P>0.05, c2 = 0.002, 3.447). The positive expression of GST-Pi in HCC (89.6%) or LHN (71.1%) was significantly higher than that in LC (22.9%, P<0.001, c2 = 49.91, 16.96). There was a significant difference between HCC and LHN (P<0.05, c2 = 6.353).
CONCLUSION: The c-erbB-2 expression is an early event in the pathogenesis of HCC. GST-Pi may be a marker enzyme for immunohistochemical detection of human HCC and its preneoplastic lesions. LHN seems to be a preneoplastic lesion related to hepatocarcinogenesis.
- Citation: Niu ZS, Wang M. Expression of c-erbB-2 and glutathione S-transferase-pi in hepatocellular carcinoma and its adjacent tissue. World J Gastroenterol 2005; 11(28): 4404-4408
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4404
Primary hepatocellular carcinoma (HCC) is one of the most common malignant tumors in Asia, and the incidence and mortality of HCC show a tendency to rise year by year. Therefore, the detection of preneoplastic lesions of HCC is crucial for the analysis of carcinogenic processes and developing strategies for prevention and treatment. For many years, liver hyperplastic nodule (LHN) has been considered as a preneoplastic lesion. Our previous studies have also confirmed that LHN is closely related to human HCC.
In the present study, the immunohistochemical LSAB method was used to detect the expression of c-erbB-2 oncogene and glutathione S-transferase-pi (GST-pi) in HCC and pericarcinomatous tissues, in order to investigate the possible roles of these genes in the HCC carcinogenesis, and to find out the relationship between LHN, liver cirrhosis (LC), and HCC.
HCC specimens from 100 patients were selected during surgical resections or biopsies performed at the Affiliated Hospital of Medical College of Qingdao University, China. Of these patients, 76 were males and 24 females with an average age of 50.4 years. None of the patients received chemo- or radiotherapy before resection. We randomly selected 41 and 77 cases of HCC to detect the expression of c-erbB-2 and GST-pi, respectively. The same specimens from 16 cases were detected for both c-erbB-2 and GST-pi, which were too few to be analyzed in terms of the correlation between them in the present study. Forty-one specimens for detecting c-erbB-2 oncogene contained 18 pericarcinomatous tissues, including 14 LHN, 17 LC. Seventy-seven specimens for detecting GST-pi contained 40 pericarcinomatous tissues, including 38 LHN, and 35 LC.
All specimens were routinely processed, alcohol-fixed and paraffin-embedded. Serial paraffin sections of 4 mm thickness were cut and used for hematoxylin and eosin and immunohistochemical staining. Immunohistochemical LSAB method was used to detect c-erbB-2 and GST-pi. Anti-c-erbB-2 multiple clonal antibody, anti-GST-pi multiple clonal antibody and LSAB kits were purchased from Dako Co. Before staining, the sections were microwave heated in 0.05 mol citric acid solution for antigen retrieval. In each staining run, a known c-erbB-2 or GST-pi positive section was added as positive control, PBS was used as substitutes of the first antibodies for negative control.
When brown granules were found on cell membrane of liver cells and cancer cells, c-erbB-2 was identified positive. When only cytoplasm was stained brown, c-erbB-2 was identified negative. The c-erbB-2 expression was graded semi-quantitatively according to the intensity and the percentage of positivity into negative (-): no positive cells or a weak staining of c-erbB-2 with positive cells<5%; positive (+): c-erbB-2 expression was relatively stronger with the positive cells>5%. For GST-pi, cells with brown granules in cytoplasm were regarded as positive. The criteria of the evaluation of GST-pi expression in this study were as follows. The positive number was semiquantitatively evaluated by counting that in 5-10 randomly chosen medium power (×100 magnification), and the three grades for GST-pi were considered as negative (-): no positive cells; positive (+): the positive reaction being brown and the positive cells <30%; strong positive (++): the positive reaction being brown and the positive cells>30% according to the intensity and extent of GST-pi staining.
Results were analyzed by c2-test and direct probability calculation. P<0.05 was considered statistically significant.
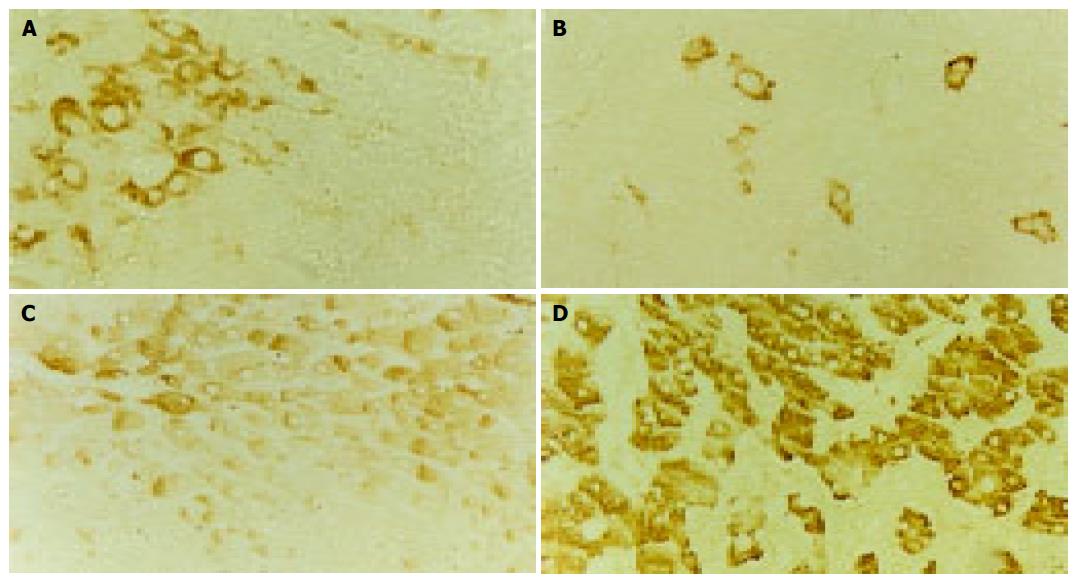
The positive staining for c-erbB-2 in cancer cells was exclusively located in cell membrane stained brown, the expression of c-erbB-2 was observed in both cell membrane and cytoplasm in one of four cases of LHN expressing c-erbB-2 (Figure 1A). The c-erbB-2 positive rate was significantly higher in LHN (28.6%) than that in LC (0%, P value 0.032, direct probability calculation, P<0.05), but no significant difference was seen between HCC (Figure 1B) and LHN or LC (c2 values 0.002, 3.447, P>0.05, Table 1).
The positive staining for GST-pi appeared as brown granules, which was predominantly located in the cytoplasm, and the staining of the nuclei was seen in part of the cancer cells. There was strong staining of GST-pi in bile duct epithelial cells. A weak staining of GST-pi was observed in LC with a positive rate of 22.9% (8/35). GST-pi expression was markedly stronger in HCC or LHN (Figure 1C). The rates of positivity were 89.6% (69/77) and 71.1% (27/38), respectively, and significantly higher than that in LC (c2 values 49.91, 16.96, P<0.001). There was also a significant difference between HCC (Figure 1D) and LHN (c2 value 6.353, P<0.05, Table 2).
The c-erbB-2/neu is a transforming proto-oncogene encoding a transmembrane glycoprotein of 185 ku (P185) with tyrosine kinase activity and extensive sequence homology to epidermal growth factor receptor. The c-erbB-2 is in a state of inactivation under normal condition, and is involved in regulating cell growth and proliferation[1]. Having been affected by some endogenous or extraneous carcinogens, its structure or expression is dysregulated, thus being activated, and c-erbB-2 becomes oncogene with tumor transformation activity. In human malignancies, the activation of c-erbB-2 is most frequently caused by gene amplification, there is extremely high concordance between copy number of gene amplification and protein overexpression[2,3]. The c-erbB-2 is frequently overexpressed in different tumors in humans, and the activation of c-erbB-2 appears to be an early event in tumorigenesis for some cancers[4-7]. However, there seems to be conflicting reports concerning the role of c-erbB-2 oncogene in carcinogenesis of HCC. Some studies reported that c-erbB-2 does not play a role in tumorigenesis of HCC[7], whereas others reported that the overexpression of c-erbB-2 is found in the middle stage of hepatocarcinogenesis, and might only have promoting effects during the development of this lesion[8]. In the present study, c-erbB-2 overexpression was observed in 24.4% (n = 41) of HCC tissue specimens and 22.2% (n = 18) of its adjacent tissue specimens, suggesting that the overexpression of c-erbB-2 oncogene plays an initial role in hepatocarcinogeneis, and is an early molecule change in the carcinogenesis of HCC. In addition, the expression of c-erbB-2 did not decrease in the transition from pericarcinomatous tissues into HCC in the present study, indicating that c-erbB-2 expression also occurs later in the process of hepatocarcinogeneis and maintains the transformed hepatocyte phenotype. Apparently, c-erbB-2 expression can contribute to the initiation and progression of HCC.
Many studies have demonstrated that c-erbB-2 gene product not only contributes to the development and maintenance of malignant phenotype, but also plays a pivotal role in transformation and tumorigenesis[4,5,9], because 3T3 cells or immortalized cells transfected with c-erbB-2 oncogene show a highly transformed and tumorigenic phenotype[10,11]. It can be inferred from these studies that those cells overexpressing c-erbB-2 oncogene are either cancerous or have malignant phenotype. In the present study, all four cases overexpressing c-erbB-2 oncogene in pericarcinomatous tissues were LHN, thus, the positive expression rate of c-erbB-2 in LHN was 28.6% (n = 14), and similar to that of HCC, suggesting that those cells overexpressing c-erbB-2 oncogene in LHN have at least acquired the malignant phenotype, which may reflect the alterations in different biologic state of LHN. Some studies reported that the increased oncogene expression brings cells into a state of active proliferation that results in an increased frequency of mutation[12]. Therefore, it is reasonable to postulate that the overexpression of c-erbB-2 oncogene may make LHN transform malignantly, and parts of LHN are actually in the preneoplastic state or might be cancerous. In contrast to LHN, the expression of c-erbB-2 oncogene is negative in LC, implying that there is no activation of c-erbB-2 in LC, i.e., it is impossible in this situation for malignant transformation. There is also no mutation of p53 in LC, consequently, LC does not necessarily link with hepatocarcinogenesis. Our findings further confirm the notion that it is not LC but LHN, is a preneoplastic lesion for the occurrence of HCC in humans.
GSTs play an important role in protecting cells against cytotoxic and carcinogenic agents. GST-pi is an acid GST, isolated and purified from human term placenta, which possesses catalytic and ligand-binding properties, and is regarded as a new marker enzyme for tumors. Some studies reported that GST-pi or GST-pi mRNA is hardly detectable in normal liver, but markedly increases in preneoplastic hepatic lesions such as hyperplastic nodules and in HCC by immunohistochemical staining or in situ hybridization[13-16], suggesting that GST-pi is a sensitive marker enzyme for preneoplastic lesions and neoplastic cells, not only at the protein level but also at the mRNA level, throughout the hepatocarcinogenesis in rat liver. In addition, other studies reported that single cells immunohistochemically positive for GST-pi induced in the rat liver carcinogenesis by chemical carcinogens are precursor-initiated cells of preneoplastic foci, GST-pi content in the single cells is higher than that in preneoplastic foci, and GST-pi is a more sensitive marker for detection of HCC than g-GT or AFP[17-20]. Therefore, it has been generally considered that GST-pi is the most accurate marker enzyme for detection of the very early “initiated cells” in chemically induced hepatocarcinogenesis in rats. It is known that human GST-pi has highly immunological cross-reaction with rat GST-pi, and the alteration of GST-pi precedes that of cell morphology. Studies in human HCC are not as advanced as in rats but have revealed close similarities. To investigate the relationship between GST-pi and HCC and its preneoplastic lesions, accordingly, may offer an important enzyme for the early diagnosis of HCC.
The expression of GST-pi might increase abnormally in the course of the carcinogenesis of many tumors, and it has been associated with preneoplastic and neoplastic changes[21,22]. Recently, many researches demonstrated that loss of transcription activity of GST-pi gene promoter in human malignancies appears to be the result of CpG island DNA methylation, and this phenomenon is most frequent in breast and renal carcinomas, and might contribute to the carcino-genetic process in these two carcinomas, while other tumor types show GST-pi promoter methylation only rarely or not at all[21-23]. However, some studies reported that GST-pi promoter hypermethylation changes occur frequently in human HCC, suggesting that somatic GST-pi inactivation via CpG island hypermethylation might contribute to the pathogenesis of HCC[24-27]. When cells display complete GST-pi hypermethylation in the CpG island, and they fail to express GST-pi mRNA and the corresponding protein product, which is different from our findings. GST-pi expression was present in 89.6% of HCC and in 71.1% of LHN in the present study. There are several possibilities for this (1) There is rare or no GST-pi promoter hypermeth-ylation in pathogenesis of HCC. (2) There is GST-pi promoter hypermethylation, but loss of GST-pi promoter hypermethylation in HCC may be due to the emergence of tumor subclones unmethylated at the GST-pi promoter during HCC transformation. Such subclones may gain additional genetic lesions that rendered GST-pi inactivation no longer necessary for neoplastic cell survival. (3) There is GST-pi promoter hypermethylation, but it is not responsible for GST-pi expression, it is the p53 gene mutation existing in LHN and HCC that activates GST-pi. p53 mutation is frequently observed in Asian HCC, but it is not a common event in Western HCC, thus, it is not difficult to interpret the reason why GST-pi is overexpressed in some studies whereas GST-pi is absent in other studies though GST-pi promoter hypermethylation may exist in pathogenesis of HCC. (4) GST-pi promoter hypermethylation is not a frequent event in pathogenesis of HCC, it may relate to the different etiologies of HCC in different geographical areas.
Markedly increased GST-pi content has been found in HCC investigated, indicating that HCC cells have characteristics of some fetal cells, as GST-pi content in fetal cells is noticeably higher than that in adult cells. It can be preliminarily inferred that GST-pi has relevance to detoxification of liver in pathogenesis of HCC. Meanwhile, the high expression of GST-pi in HCC might be associated with the resistance to anticancer drugs and might protect the tumor cells themselves against the cytotoxic effects of free radicals, as described in other tumors[28-31]. It is well-known that GST-pi can detoxify not only electrophiles derived from xenobiotics, but also endogenous electrophiles usually with the consequence of free radical damage. These data indicate that the overexpression of GST-pi may impart a proliferative advantage in HCC cells due to induction of resistance to several different cytotoxic mechanisms. In the present study, the positive rate of GST-pi in HCC was higher than that in pericarcinomatous tissues, and was significantly higher in LHN than in LC, showing that GST-pi is activated during initiation stage or much earlier stage of cell transformation induced by chemical carcinogens, and may be acquired in increased amounts during malignant progression. Therefore, the data obtained in the present study suggest that GST-pi may be an effective marker enzyme for HCC and its preneoplastic lesions.
As an important enzyme of detoxification, GST-pi protects cells against the influence of carcinogenic materials. However, GST-pi is taken as a double-edged sword in tumorigenesis, namely, GST-pi protects all cells expressing it, including normal cells, cells with malignant phenotype, and tumor cells as well. Many cells in LHN are in a state of active proliferation, and have acquired the malignant phenotype, thus GST-pi evolves specifically to protect these proliferating cells, and these cells inevitably proliferate rapidly, presenting a clonal expansion. Our findings suggest that cells overexpressing GST-pi in LHN may relate to the carcinogenesis of LHN, and some cells in LHN are altered hepatocytes, which should be treated as preneoplastic cells of HCC. In contrast to LHN, the cells in LC appear to represent quiescent phenotypes unlikely to progress into HCC.
| 1. | Zhou BP, Hung MC. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin Oncol. 2003;30:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Bánkfalvi A. [HER-2 diagnostics]. Magy Onkol. 2002;46:11-15. [PubMed] |
| 3. | Nathanson DR, Culliford AT, Shia J, Chen B, D'Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB. HER 2/neu expression and gene amplification in colon cancer. Int J Cancer. 2003;105:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Lazar H, Baltzer A, Gimmi C, Marti A, Jaggi R. Over-expression of erbB-2/neu is paralleled by inhibition of mouse-mammary-epithelial-cell differentiation and developmental apoptosis. Int J Cancer. 2000;85:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol. 2001;28:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Bewick M, Lafrenie RM. EGFR and ErbB2 differentially regulate Raf-1 translocation and activation. Lab Invest. 2002;82:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Vlasoff DM, Baschinsky DY, De Young BR, Morrison CD, Nuovo GJ, Frankel WL. C-erb B2 (Her2/neu) is neither overexpressed nor amplified in hepatic neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Shi G, Sun C, Han X, Meng X, Wang M, Gu M. Expression of rasp21, C-myc, C-erbB-2, and AFP in 2-FAA induced experimental hepatocarcinogenesis. Zhonghua Gan Zang Bing Za Zhi. 2001;9:98-99. [PubMed] |
| 9. | Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation. Ann Oncol. 2001;12 Suppl 1:S9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Yu D, Hamada J, Zhang H, Nicolson GL, Hung MC. Mechanisms of c-erbB2/neu oncogene-induced metastasis and repression of metastatic properties by adenovirus 5 E1A gene products. Oncogene. 1992;7:2263-2270. [PubMed] |
| 11. | Kusakari T, Kariya M, Mandai M, Tsuruta Y, Hamid AA, Fukuhara K, Nanbu K, Takakura K, Fujii S. C-erbB-2 or mutant Ha-ras induced malignant transformation of immortalized human ovarian surface epithelial cells in vitro. Br J Cancer. 2003;89:2293-2298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lian ZR. HBV status and expression of ets-2, IGF-II, C-myc and N-ras in human hepatocellular carcinoma and adjacent nontumorous tissues--a comparative study. Zhonghua Zhong Liu Za Zhi. 1991;13:5-8. [PubMed] |
| 13. | Sawaki M, Hattori A, Tsuzuki N, Sugawara N, Enomoto K, Sawada N, Mori M. Chronic liver injury promotes hepatocarcinogenesis of the LEC rat. Carcinogenesis. 1998;19:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Nishikawa T, Wanibuchi H, Ogawa M, Kinoshita A, Morimura K, Hiroi T, Funae Y, Kishida H, Nakae D, Fukushima S. Promoting effects of monomethylarsonic acid, dimethylarsinic acid and trimethylarsine oxide on induction of rat liver preneoplastic glutathione S-transferase placental form positive foci: a possible reactive oxygen species mechanism. Int J Cancer. 2002;100:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Shukla Y, Arora A. Enhancing effects of mustard oil on preneoplastic hepatic foci development in Wistar rats. Hum Exp Toxicol. 2003;22:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Imai T, Masui T, Ichinose M, Nakanishi H, Yanai T, Masegi T, Muramatsu M, Tatematsu M. Reduction of glutathione S-transferase P-form mRNA expression in remodeling nodules in rat liver revealed by in situ hybridization. Carcinogenesis. 1997;18:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Satoh K, Itoh K, Yamamoto M, Tanaka M, Hayakari M, Ookawa K, Yamazaki T, Sato T, Tsuchida S, Hatayama I. Nrf2 transactivator-independent GSTP1-1 expression in "GSTP1-1 positive" single cells inducible in female mouse liver by DEN: a preneoplastic character of possible initiated cells. Carcinogenesis. 2002;23:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Satoh K, Hatayama I. Anomalous elevation of glutathione S-transferase P-form (GST-P) in the elementary process of epigenetic initiation of chemical hepatocarcinogenesis in rats. Carcinogenesis. 2002;23:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Tatematsu M, Mera Y, Inoue T, Satoh K, Sato K, Ito N. Stable phenotypic expression of glutathione S-transferase placental type and unstable phenotypic expression of gamma-glutamyltransferase in rat liver preneoplastic and neoplastic lesions. Carcinogenesis. 1988;9:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Yusof YA, Yan KL, Hussain SN. Immunohistochemical expression of pi class glutathione S-transferase and alpha-fetoprotein in hepatocellular carcinoma and chronic liver disease. Anal Quant Cytol Histol. 2003;25:332-338. [PubMed] |
| 21. | Esteller M, Corn PG, Urena JM, Gabrielson E, Baylin SB, Herman JG. Inactivation of glutathione S-transferase P1 gene by promoter hypermethylation in human neoplasia. Cancer Res. 1998;58:4515-4518. [PubMed] |
| 22. | Miyanishi K, Takayama T, Ohi M, Hayashi T, Nobuoka A, Nakajima T, Takimoto R, Kogawa K, Kato J, Sakamaki S. Glutathione S-transferase-pi overexpression is closely associated with K-ras mutation during human colon carcinogenesis. Gastroenterology. 2001;121:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427-5440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 900] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Zhong S, Tang MW, Yeo W, Liu C, Lo YM, Johnson PJ. Silencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomas. Clin Cancer Res. 2002;8:1087-1092. [PubMed] |
| 25. | Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. J Biol Chem. 2002;277:22573-22580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Yang B, Guo M, Herman JG, Clark DP. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol. 2003;163:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 288] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 28. | Ruscoe JE, Rosario LA, Wang T, Gaté L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1-1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther. 2001;298:339-345. [PubMed] |
| 29. | Goto S, Kamada K, Soh Y, Ihara Y, Kondo T. Significance of nuclear glutathione S-transferase pi in resistance to anti-cancer drugs. Jpn J Cancer Res. 2002;93:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Hara T, Ishii T, Fujishiro M, Masuda M, Ito T, Nakajima J, Inoue T, Matsuse T. Glutathione S-transferase P1 has protective effects on cell viability against camptothecin. Cancer Lett. 2004;203:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Chandra RK, Bentz BG, Haines GK, Robinson AM, Radosevich JA. Expression of glutathione s-transferase pi in benign mucosa, Barrett's metaplasia, and adenocarcinoma of the esophagus. Head Neck. 2002;24:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK