Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4332
Revised: November 1, 2004
Accepted: November 4, 2004
Published online: July 28, 2005
AIM: Tumor angiogenesis has been shown to be promoted by vascular endothelial growth factor (VEGF) via stimulating endothelial cell proliferation, migration, and survival. Blockade of VEGF signaling by different means has been demonstrated to result in reduced tumor growth and suppression of tumor angiogenesis in distinct tumor entities. Here, we tested a recombinant adenovirus, AdsFlt1-3, that encodes an antagonistically acting fragment of the VEGF receptor 1 (Flt-1), for systemic antitumor effects in pre-established subcutaneous CRC tumors in mice.
METHODS: Murine colorectal carcinoma cells (CT26) were inoculated subcutaneously into Balb/c mice for in vivo studies. Tumor size and survival were determined. 293 cell line was used for propagation of the adenoviral vectors. Human lung cancer line A549 and human umbilical vein endothelial cells were transfected for in vitro experiments.
RESULTS: Infection of tumor cells with AdsFlt1-3 resulted in protein secretion into cell supernatant, demonstrating correct vector function. As expected, the secreted sFlt1-3 protein had no direct effect on CT26 tumor cell proliferation in vitro, but endothelial cell function was inhibited by about 46% as compared to the AdLacZ control in a tube formation assay. When AdsFlt1-3 (5×109 PFU/animal) was applied to tumor bearing mice, we found a tumor inhibition by 72% at d 12 after treatment initiation. In spite of these antitumoral effects, the survival time was not improved. According to reduced intratumoral microvessel density in AdsFlt1-3-treated mice, the antitumor mechanism can be attributed to angiostatic vector effects. We did not detect increased systemic VEGF levels after AdsFlt1-3 treatment and liver toxicity was low as judged by serum alanine aminotransferase determination.
CONCLUSION: In this study we confirmed the value of a systemic administration of AdsFlt1-3 to block VEGF signaling as antitumor therapy in an experimental metastatic colorectal carcinoma model in mice.
- Citation: Schmitz V, Kornek M, Hilbert T, Dzienisowicz C, Raskopf E, Rabe C, Sauerbruch T, Qian C, Caselmann WH. Treatment of metastatic colorectal carcinomas by systemic inhibition of vascular endothelial growth factor signaling in mice. World J Gastroenterol 2005; 11(28): 4332-4336
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4332
Colorectal carcinoma (CRC) is one of the most common malignant diseases in the Western countries. Even after successful resection of the primary tumor, about one-third of the patients develop tumor recurrence. The most common place of distant CRC metastases are liver and lung[1]. In particular, metastatic CRC disease is associated with limited life expectancy and progressive tumor disease in CRC is associated with increased VEGF levels[2]. Considering clinical studies successfully employing tyrosine kinase inhibitors for systemic tumor therapy[3] and experimental studies showing the antitumor efficacy of VEGF-antagonism in a pancreatic adenocarcinoma animal model[4] and follicular thyroid carcinoma animal model[5], including successful local treatment of subcutaneous CRC[6], we evaluated and confirmed the antitumoral efficacy and mechanism of a systemic gene delivery of a Flt-1 fragment (sFlt1-3) in subcutaneous metastatic CRC in mice.
Vascular endothelial growth factor (VEGF) expression correlates with tumor vascularization in most tumor types, also in CRC[2,7,8]. VEGF binds with different affinity to its cell surface receptors, VEGF receptor 1 (Flt-1), VEGF receptor 2 (Flk-1) and VEGF receptor 3 (Flt-4). All three VEGF receptors are members of the class III receptor-type tyrosine kinase receptor family[9]. Several findings suggest that binding of VEGF to Flt-1 regulates angiogenesis by controlling inter-cellular endothelial interactions[10].
Since angiostatic therapies do not attack the malignant tumor cell itself, but address tumor vascularization, a systemic treatment is of particular interest. Therefore, we investigated the effect of interruption of the VEGF cascade by systemic gene delivery on pre-established tumors.
Balb/c mice, 6 wk old, were purchased from Charles River (Sulzfeld, Germany) and kept in the local central animal facility. The mice were housed under standard conditions and had free access to water and food. Animal procedures were performed in accordance to approved protocols and followed recommendations for proper care and use of laboratory animals.
For propagation of adenoviral vectors, 293 cells (embryonic E1 transformed kidney cell line) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) Cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS).
To demonstrate vector function and gene expression standard techniques using the human lung cancer cell line A549(ATCC, Manassas, VA, USA) were employed for in vitro transfection experiments. Cells were cultured in DMEM supplemented with 10% heat-inactivated FBS. Human umbilical vein endothelial (HUVE) cells were obtained from Cascade Biologics (Portland, OR, USA) and were cultured according to the supplier's instructions.
Murine CRC CT26 cells have originally been described by Brattain[11]. Cells were cultured in DMEM supplemented with 10% heat-inactivated FCS and 1% penicillin/streptomycin.
The recombinant adenoviruses encoding the LacZ-gene was constructed as described previously[12]. The AdsFlt1-3 construct was generously provided by R. Mulligan, Boston, MA, USA, it consists of the extracellular immunoglobulin-like domains 1-3 of the VEGF-receptor 1 (Flt-1) and has a His-tag; its construction has been described elsewhere[13]. Recombinant adenoviruses were expanded, and purified by double cesium-chloride ultra-centrifugation. Purified viruses were dialyzed against 10 mmol/L Tris/1 mmol/L MgCl2 and stored in glycerol aliquots at -80 °C. Virus concentrations were determined by measuring virus particles (opu/mL) and by cytotoxic plaque assay (pfu/mL) in 293 cells. Virus productions were tested for wild-type adenovirus contamination by cytotoxic plaque assay in A549 cells.
A549 tumor cells were transfected with 250 multiplicity of infection (MOI) of AdLacZ or AdsFlt1-3. Forty-eight hours later, cells and culture medium (CM) were harvested. RNA was isolated from the using the GenElute Total Mammalian RNA Kit (Sigma, Taufkirchen, Germany) according to the manufacturer's protocol. RNA was then digested with RQ1 DNase (Promega, Mannheim, Germany). RNA concentrations were determined and 1 mg RNA was used in the RT reaction with random primers (Promega, Mannheim, Germany) and MMLV reverse transcriptase (Promega, Mannheim, Germany). PCR for sFlt1-3 was performed with the forward primer 5'-CGTTCCAGTCTTTCAACACC-3' and reverse primer 5'-CCAAGGAAACGTGAAAGC-3'. As positive control, primers for human b-actin were used. The size of the amplified product was about 250 bp. Analysis of the PCR products occurred by electrophoresis on a 1% agarose gel.
For detection of sFlt1-3 100 mL of the harvested CM was dispensed in a 96-well ELISA plate and incubated at 4 °Covernight. After washing thrice with PBS, the ELISA plate was incubated for 2 h with anti mouse VEGF-R1 (Flt-1) antibody (1:200, R&D Systems, Wiesbaden-Nordenstadt, Germany). After antibody incubation, the plate was washed with PBS and incubated with secondary antibody (1:25 000, monoclonal anti-sheep/goat peroxidase conjugate, Sigma, St. Louis, MO, USA) 2 h. After washing with PBS the plate was incubated for 15 min with TMB substrate (Biozol, Eching, Germany). Color intensity was measured in an ELISA reader (Dynatech Laboratories, Frankfurt, Germany).
Five thousand CT26 cells were resuspended in 40 mL of culture medium and dispensed in each well of a 96-culture plate and pre-incubated with 50 mL of CM. After 30 min of pre-incubation time, 150 mL of RPMI 1640 containing 10% FCS was added. Cell culture was continued for 18 h and then cells were labeled with BrdU for further 24 h. The BrdU assay was performed according to the manufacturer's protocol (BrdU proliferation assay, Roche Diagnostics, Mannheim, Germany).
A 24-well plate was coated with 300 mL Matrigel (BD Biosciences, Bedford, MA, USA). Twenty-four hours later, HUVE cells (passage number <10) in 75 mL Medium200 (2.5×104 cells) were seeded on the Matrigel and pre-incubated for 30 min with 75mL of CM (derived from Huh7 cells). One hundred and fifty microliters of Medium200 was added and the cells were additionally incubated for 4-6 h. Tube-like formations were counted under the light microscope in high power fields.
In vivo antitumoral efficacies were studied in a subcutaneous CRC mouse model in C3H mice. 106 CT26 cells were resuspended in 100 mL FCS-free culture medium and injected subcutaneously via a 28-G syringe.
When reaching a tumor volume of 40 mm3, tumor treatment was initiated by intravenous injection of 5×109 pfu/animal AdLacZ (n = 11) and AdsFlt1-3 (n = 10) in 150 mL NaCl. Tumor volumes were calculated by the formula: v = length ×width20.52. From a subgroup of animals treated intravenously with AdLacZ (n = 2), or AdsFlt1-3 (n = 3) serum and tumor samples were taken at different time points (at d 3, 6, 9, 14, 19, and 25) for VEGF-ELISA and histology, respectively.
Tumor samples were embedded in tissue tech (DAKO), snap frozen in liquid nitrogen and stored at -80 °C for anti-von Willebrand staining. Five millimeters of cryostat sections were gently warmed up at RT. Then fixed in acetone for 10 min and dried on air. Sections were stained with dilution of primary antibody (polyclonal rabbit antihuman von Willebrand factor, 1:1 600). After washing with PBS, sections were incubated with secondary antibody dilution of biotinylated pig antirabbit immunoglobulin G (1:300) and streptavidin conjugated to horseradish peroxidase (DAKO). Sections were visualized by using the Dako ChemMate detection kit and counterstained with hematoxylin (DAKO).
All measured data are given with mean±SE. Differences between values of different experimental groups were analyzed for statistical significance by a non-parametric, two-tailed test (Mann-Whitney test) for unpaired samples and in case of histology by unpaired Student's t-test. Survival rates are presented as Kaplan-Maier curves. An error level P <0.05 was supposed to indicate significance.
Wild-type contamination of the adenoviral stock solutions was analyzed in A549 cells in a cytotoxic plaque assay. Virus concentrations were determined as optical particle units and ranged from 2.13×1012 opu/mL for AdLacZ to 7.04×1012 opu/mL for AdsFlt1-3, respectively. Virus titration in 293 cell plaque assays showed corresponding concentrations of 8.0×1012 pfu/mL for AdLacZ and 1.027×1012 pfu/mL for AdsFlt1-3.
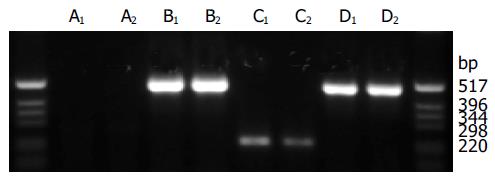
CM of infected A549 cells was harvested to demonstrate sFlt1-3 gene expression and protein secretion into cell supernatant. The analysis of the RT-PCR showed similar bands for b-actin (500 bp) in all samples and only AdsFlt1-3 infected cells showed an extra band at about 250 bp representing the encoded transgene sFlt1-3 (Figure 1). Protein expression was confirmed by ELISA in AdsFlt1-3 infected cells (106 ng/mL). This data demonstrate correct vector function and protein secretion.
To check for direct effects of sFlt1-3 on CRC tumor cells, conditioned CM was tested for antitumor effects on CT26 tumor cells. As expected, we did not observe any direct anti-proliferative effects (data not shown). We concluded that potential antitumor effects were mediated by indirect (angiostatic) mechanism and not by cytotoxic effects.
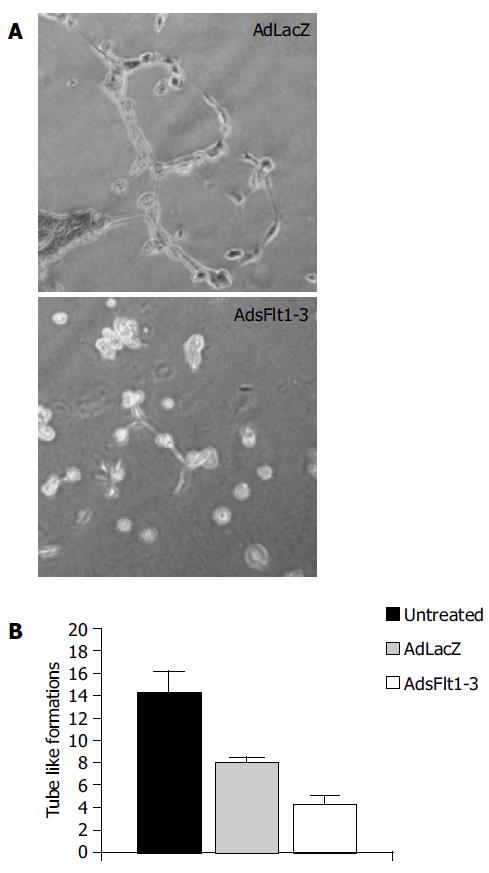
The tube formation assay tests the capability of HUVE cells to form tube-like structures comprising endothelial cell function like migration and tube formation. The results showed that sFlt1-3 inhibited these cell functions by about 46% (Figure 2) compared to the AdlacZ control. This indicates biological activity of sFlt1-3 in vitro.
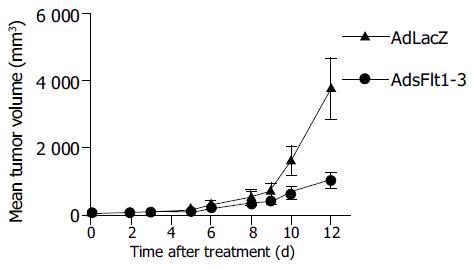
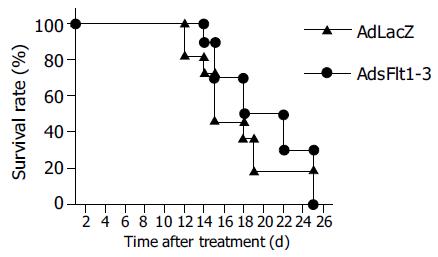
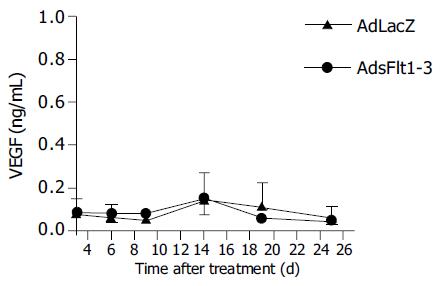
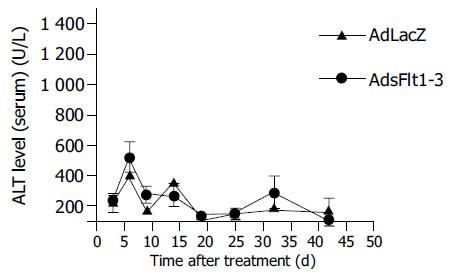
Since CT26 tumor cells express and secrete VEGF (68 pg/mL) into cell supernatant, the CT26 tumor model is suitable to study antitumor effects of an anti-VEGF therapy. The systemic injection of 5×109 pfu/mL AdsFlt1-3 significantly reduced tumor growth by 72% compared to the AdLacZ control 12 d after treatment initiation (Figure 3), but did not improve the survival rate (Figure 4). VEGF serum levels were similar in both groups, AdLacZ and AdsFlt1-3, (Figure 5) and we did not observe any compensatory upregulation of VEGF levels in serum. Liver toxicity was tolerable according to alanine aminotransferase levels in serum (Figure 6).
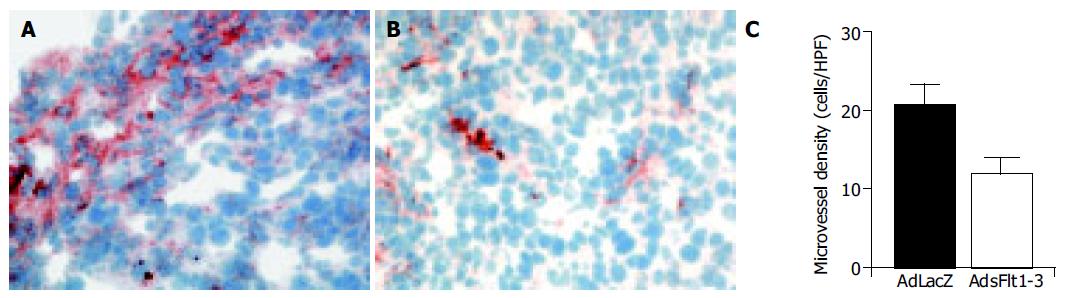
Intratumoral microvessels density was determined by immunohistochemistry for von Willebrand factor. Tumors were removed 9 d after treatment and as shown in Figure 7, tumors of animals that had received AdLacZ showed intense staining for von Willebrand factor, indicating effective tumor vascularization, and tumor sections of AdsFlt1-3-treated animals showed a marked reduction by 42% in microvessel density (Figure 7).
VEGF has been acknowledged as one of the most important pro-angiogenic factors that are known to be involved in tumor angiogenesis[7,14-16]. In particular, VEGF expression has been shown to be elevated in metastatic CRC disease[2]. In the case of metastatic CRC, therapeutic options are still limited and innovative approaches are warranted. Therefore, we intended to confirm the antitumor efficacy of blocking VEGF signaling by systemic administration of an adenovirus delivering the VEGF receptor 1 fragment sFlt1-3 in a subcutaneous metastatic murine CRC tumor model. Our data show that the systemic vector application of AdsFlt1-3 induced significant antitumor effects in CRC.
Recently, several angiostatic antitumor approaches have been used to inhibit experimental CRC. In a previous publication, we were able to show that the local gene delivery of angiostatin-like molecule (K1-3) inhibited tumor growth in the same CRC model by 41% at d 8 after treatment initiation. Another group had used a similar approach employing an adenoviral construct encoding a distinct Flt-1 fragment to treat subcutaneous tumors, but then only the local treatment was effective[6]. But principally, angiostatic antitumor strategies do not intend to directly control growth of the malignant tumor cell but rather to curb tumor vascularization. Therefore, systemic gene delivery of angiostatic proteins might even be more effective than local applications. The vector construct we used in this study had been shown to inhibit e.g. tumor growth of fibrosarcoma and lung cancer in experimental studies after systemic vector administration[13]. Here, we were able to confirm the antitumoral efficacy of the AdsFlt1-3 construct in a metastatic subcutaneous CRC model. But, according to other angiostatic gene therapy studies and own findings, the survival rate was not improved.
Since we could not observe any direct antiproliferative effects of sFlt1-3 on CT26 CRC cells, antitumoral effects most probably were mediated by indirect angiostatic effects. This was supported by immune staining for von Willebrand factor revealing decreased microvessel density in the AdsFlt1-3 treatment group. Taken together, these results correspond well to other publications reporting anti-angiogenic effects of similar constructs in corneal or ocular neovascularization assays[13].
Recently, potential side effects of adenoviruses have provoked severe concerns of systemic vector applications regarding particularly liver toxicity. Indeed, we observed some ALT elevations, but at the dosage applied toxicity was well tolerated.
In summary our data demonstrate that the systemic gene transfer of sFlt1-3 induced significant antitumor effects on pre-established subcutaneous metastatic CRC and antitumor mechanism based on angiostatic effects. Thus, these data further confirmed the value of blocking VEGF signaling as an effective approach to treat CRC disease.
AdsFlt1-3 construct was generously provided by R. Mulligan, Boston, MA, USA. We thank I. Hoschler for her expert assistance.
| 1. | Ohlsson B, Pålsson B. Follow-up after colorectal cancer surgery. Acta Oncol. 2003;42:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Semin Oncol. 2003;30:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Tseng JF, Mulligan RC. Gene therapy for pancreatic cancer. Surg Oncol Clin N Am. 2002;11:537-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ye C, Feng C, Wang S, Wang KZ, Huang N, Liu X, Lin Y, Li M. sFlt-1 gene therapy of follicular thyroid carcinoma. Endocrinology. 2004;145:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Kong HL, Hecht D, Song W, Kovesdi I, Hackett NR, Yayon A, Crystal RG. Regional suppression of tumor growth by in vivo transfer of a cDNA encoding a secreted form of the extracellular domain of the flt-1 vascular endothelial growth factor receptor. Hum Gene Ther. 1998;9:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853-865. [PubMed] |
| 8. | Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1807] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 9. | Brattain MG, Strobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980;40:2142-2146. [PubMed] |
| 10. | Qian C, Bilbao R, Bruña O, Prieto J. Induction of sensitivity to ganciclovir in human hepatocellular carcinoma cells by adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase. Hepatology. 1995;22:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, von Recum HA, Yuan J, Kamihara J, Flynn E. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 228] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2567] [Cited by in RCA: 2544] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 13. | Shibuya M. Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 226] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1055] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Schmitz V, Wang L, Barajas M, Gomar C, Prieto J, Qian C. Treatment of colorectal and hepatocellular carcinomas by adenoviral mediated gene transfer of endostatin and angiostatin-like molecule in mice. Gut. 2004;53:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Xiao WH, Duh EJ, Yang HS, Lai H, Kovesdi I, Carrion M. Periocular gene transfer of sFlt-1 suppresses ocular neovascularization and vascular endothelial growth factor-induced breakdown of the blood-retinal barrier. Hum Gene Ther. 2003;14:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Science Editor Zhu LH Language Editor Elsevier HK