Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4317
Revised: October 23, 2004
Accepted: October 26, 2004
Published online: July 28, 2005
AIM: To investigate the damaging effect of high-intensity focused ultrasound (HIFU) on cancer cells and the inhibitory effect on tumor growth.
METHODS: Murine H22 hepatic cancer cells were treated with HIFU at the same intensity for different lengths of time and at different intensities for the same length of time in vitro, the dead cancer cells were determined by trypan blue staining. Two groups of cancer cells treated with HIFU at the lowest and highest intensity were inoculated into mice. Tumor masses were removed and weighed after 2 wk, tumor growth in each group was confirmed pathologically.
RESULTS: The death rate of cancer cells treated with HIFU at 1 000 W/cm2 for 0.5, 1, 2, 4, 8, and 12s was 3.11±1.21%, 13.37±2.56%, 38.84±3.68%, 47.22±5.76%, 87.55±7.32%, and 94.33±8.11%, respectively. A positive relationship between the death rates of cancer cells and the length of HIFU treatment time was found (r = 0.96, P<0.01). The death rate of cancer cells treated with HIFU at the intensity of 100, 200, 400, 600, 800, and 1 000 W/cm2 for 8s was 26.313±.26%, 31.00±3.87%, 41.97±5.86%, 72.23±8.12%, 94.90±8.67%, and 99.30±9.18%, respectively. A positive relationship between the death rates of cancer cells and the intensities of HIFU treatment was confirmed (r = 0.98, P<0.01). The cancer cells treated with HIFU at 1 000 W/cm2 for 8s were inoculated into mice ex vivo. The tumor inhibitory rate was 90.35% compared to the control (P<0.01). In the experimental group inoculated with the cancer cells treated with HIFU at 1 000 W/cm2 for 0.5s, the tumor inhibitory rate was 22.9% (P<0.01). By pathological examination, tumor growth was confirmed in 8 out of 14 mice (57.14%, 8/14) inoculated with the cancer cells treated with HIFU at 1 000 W/cm2 for 8s , which was significantly lower than that in the control (100%, 15/15, P<0.05).
CONCLUSION: HIFU is effective on killing or damage of H22 hepatic cancer cells in vitro and on inhibiting tumor growth in miceex vivo.
- Citation: Wang XJ, Yuan SL, Lu YR, Zhang J, Liu BT, Zeng WF, He YM, Fu YR. Growth inhibition of high-intensity focused ultrasound on hepatic cancer in vivo. World J Gastroenterol 2005; 11(28): 4317-4320
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4317.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4317
High-intensity focused ultrasound (HIFU) consists of focused ultrasound (ULS) waves emitted from a transducer and is capable of inducing tissue damage. By means of this thermal effect and other mechanisms, HIFU-treated tumor tissues result in direct thermal cytotoxic necrosis and fibrosis, thus leading to inhibition of tumor growth. Therefore, HIFU is a new-sophisticated high-technology based minimally invasive treatment option for some cancers, which allows radiation-free treatment. Until now there are many kinds of tumors, such as tumors of prostate, liver, kidney, bladder, breast, and brain, that were treated with HIFU clinically and experimentally, some cancers were effectively controlled after HIFU treatment. As one of the minimally invasive surgical techniques for cancer treatment, HIFU is of great interest today[1,2].
Malignant cells are sensitive to therapeutic ULS treatment, which leads to a transient decrease in cell proliferation[3] through inducing a complex signaling cascade with upregulation of proapoptotic genes and downregulation of cellular survival genes[4]. In in vitro study, it was confirmed that CZ901 HIFU inhibits proliferation and induces apoptosis of cancer cells[5]. This study aimed to investigate the effects of HIFU on cancer cell damage in vitro and tumor growth inhibition ex vivo.
Cancer cell line in mouse Murine hepatoma H22 cell line was kept in liquid nitrogen for regular use in our laboratory[6].
Experimental animals Female Balb/C mice, weighing 18-22g, were purchased from Beijing Biological Products Research Institute under Ministry of Public Health (approval number: 013072). The procedures involving animals and their care were conducted in accordance with institutional guidelines for Laboratory Animal Care of Experimental Animal Center, Sichuan University.
Experimental device CZ901 HIFU device for cancer treatment was designed and supplied by Mianyang Electronic Equipment Factory.
Experiment in vitro Ascites taken from H22 hepatic cancer bearing mouse on d 8 or 9 was diluted with normal saline at 1:5 (2.5 × 107 cells/mL) and distributed into 14 PVC tubes, 7 tubes in each test, each containing 1.8 mL. Twelve tubes were treated with HIFU, and two were used as controls. H22 hepatic cancer cells were treated with HIFU at the frequency of 1.048 MHz and at the intensity of 1 000 W/cm2 for 0.5, 1, 2, 4, 8, and 12s respectively, and for 8s at intensity of 100, 200, 400, 600, 800, and 1 000 W/cm2, respectively. After HIFU treatment, the cells were incubated in a humidified atmosphere of 50 mg/mL CO2 at 37°C for 6 h, and then the viability of cancer cells was determined by exclusion of trypan blue staining. The viable cells were not stained, the dead cells were stained blue. The viable cells and dead cells were counted with an erythrocytometer under microscope, respectively (total cell number counted >1 000). The death rate was determined by dead cell number(the dead cell number+the viable cell number)100%. Each experiment was performed in triplicate.
Inoculation of HIFU-treated cancer cells ex vivo Cancer cells including viable and dead cells treated with HIFU at 1 000 W/cm2 for 8s were inoculated into 14 mice, 2× 106 cells/0.2 mL per mouse. The same number of untreated cancer cells was inoculated into 20 mice as control. In addition, cancer cells treated with HIFU at 1 000 W/cm2 for 0.5s were inoculated into 18 mice, 2× 106 cells/0.2 mL per mouse. The same number of untreated cancer cells was inoculated into 20 mice as control.
Examining the tumor growth ex vivo The animals inoculated with cancer cells were raised routinely, with free access to food and water and weighed every 2 d. After 2 wk of inoculation, the animals were killed, the tumor masses were removed and weighed, and the tumor inhibitory rate was calculated[6].
Histopathological examination Tumor masses were fixed with 4% paraformaldehyde, embedded with paraffin, sectioned and stained with HE. The tumor growth inhibition was confirmed by microscopy.
The experimental data were expressed as mean±SD and analyzed with c2 test. P<0.05 was considered statistically significant.
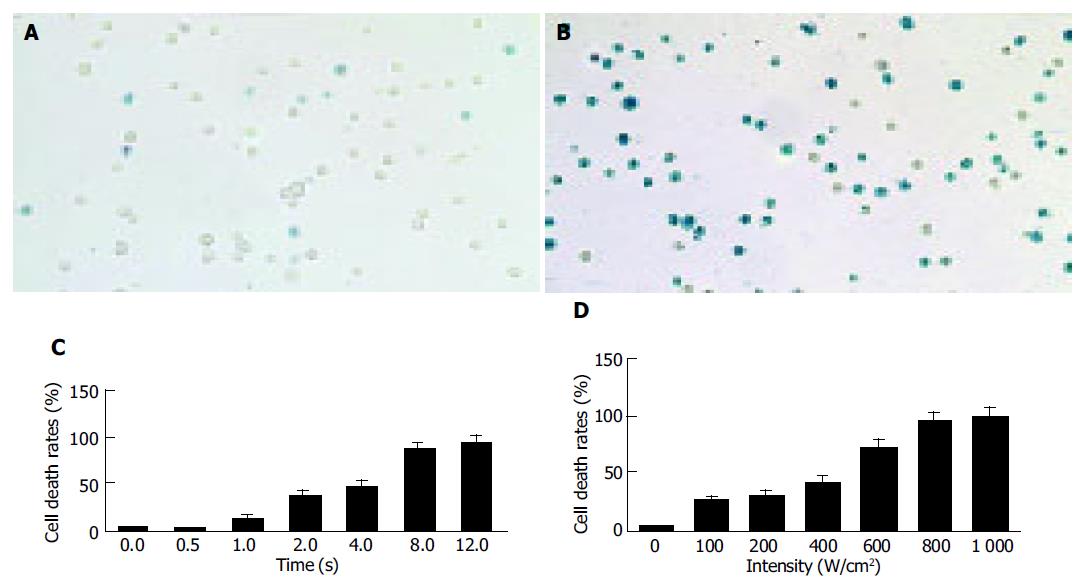
The death rate of cancer cells in controls was 3-5% (Figure 1A), and increased significantly after HIFU treatment (Figure 1B). The death rate of cancer cells treated with HIFU at 1 000 W/cm2 for 0.5, 1, 2, 4, 8, and 12s were 3.11±1.21%, 13.37±2.56%, 38.84±3.68%, 47.22±5.76%, 87.55±7.32%, and 94.33±8.11%, respectively (Figure 1C). A positive relationship was found between the death rate of cancer cells and the time of HIFU treatment (r = 0.96, P<0.01).
The death rate of cancer cells treated with HIFU at the intensity of 100, 200, 400, 600, 800, and 1 000 W/cm2 for 8s was 26.31±3.26%, 31.00±3.87%, 41.97±5.86%, 72.23±8.12%, 94.90±8.67%, and 99.30±9.18%, respectively (Figure 1D). A positive relationship was confirmed between the death rates of cancer cells and the intensities of HIFU treatment (r = 0.98,P<0.01).
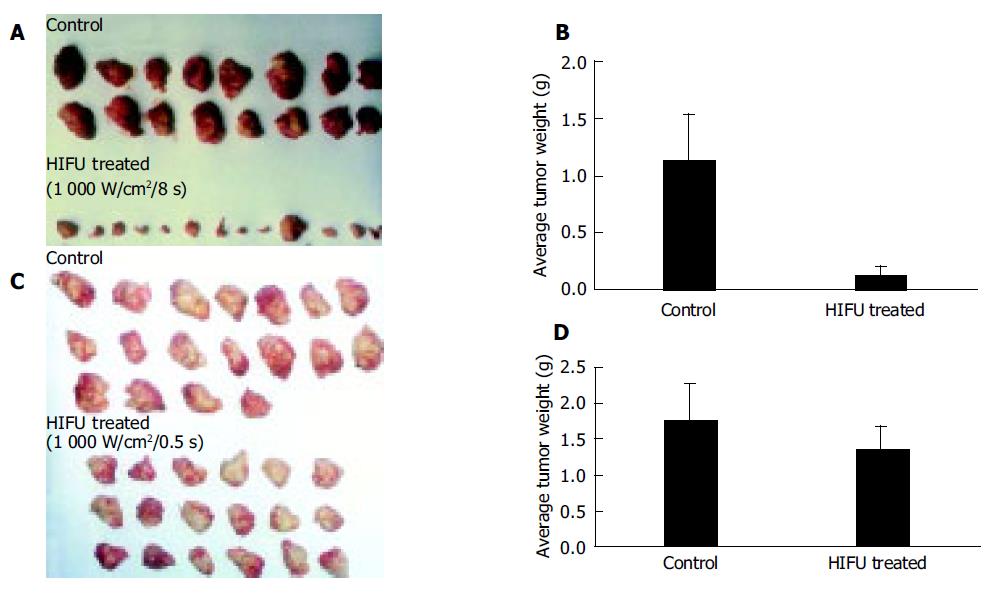
Tumor growth inhibition of cancer cells treated with HIFU ex vivo is listed in Table 1. In experiment 1, six mice in the control group died of tumor burden spontaneously, none died in the HIFU-treated group. There was no significant difference in body weight increase between two groups of animals. In the group of animals inoculated with cancer cells treated with HIFU at 1 000 W/cm2 for 8s the average tumor weight was 0.11±0.16g and the average tumor weight in control group was 1.14±0.4g (Figures 2A and B), the tumor inhibition rate was 90.35% compared to the control (P<0.01). In experiment 2, cancer cells treated with HIFU at 1 000 W/cm2 for 0.5s were inoculated. The average tumor weight in HIFU-treated group and control was 1.36±0.33 and 1.75±0.53g, respectively (Figures 2C and D), the tumor inhibitory rate was 22.90% (P<0.01).
By pathological examination, tumor growth was confirmed in 8 out of 14 mice (57.14%, 8/14) inoculated with cancer cells treated with HIFU at 1 000 W/cm2 for 8s which was significantly lower than that of the control (100%, 15/15, P<0.05).
HIFU consists of focused ULS waves emitted from a transducer and is capable of inducing tissue damage. By means of this thermal effect and other mechanisms, HIFU-treated tumor tissues resulted in direct thermal cytotoxic necrosis and fibrosis, thus leading to inhibition of tumor growth. Therefore, HIFU is a new-sophisticated high-technology based, minimally invasive treatment option for some cancers[1,4,5]. But, there are many factors affecting its therapeutic effect, such as, intensity of the transmitted pulse, the exposure time, the signal frequency, the time interval between two firing bursts, and biological medium,etc.[1,7-9].
The HIFU device used in this experimental study was designed and manufactured in Mianyang Electronic Equipment Factory. Its signal frequency emitted is 1.048 MHz, the intensity of the transmitted pulse and exposure time can be manipulated[5]. In this experimental study, H22 hepatic cancer cells were treated with HIFU at the same intensity for different lengths of time and for the same length of time at different intensities in vitro. It showed a intensity and time-dependent damaging effect on cancer cells (Figures 1B-D), suggesting that HIFU has a damaging or killing effect on cancer cells in vitro. The effective parameters are: an intensity of 1 000 W/cm2 and an exposure time of 8s.
There are many experimental and clinical studies on treatment of tumors with HIFU[1,10-14], especially hepatic cancers[15-19]. The results of these studies in vitro and in vivo indicate that HIFU has damaging or killing effect on cancer cells in vitro and inhibitory effect on tumor growth in vivo. However, to our best knowledge, there is no study on the growth potential of cancer cells after HIFU treatment. The findings in this study indicate that most cancer cells treated with HIFU at 1 000 W/cm2 would die and lose the proliferating potential, but few cells may survive and form tumors.
Although minimally invasive methods for the treatment of cancer, such as HIFU, and high-energy shock waves, have been proposed recently, their feasibility for treatment of human cancers needs to be confirmed[1,20]. This experimental study has confirmed that HIFU has effects on killing or damage of H22 hepatic cancer cells in vitro and on the inhibiting tumor growth in mice ex vivo. Its inhibitory and therapeutic effects on other cancers, and mechanisms of action need to be studied and confirmed further.
| 1. | Chaussy CG, Thüroff S. High-intensive focused ultrasound in localized prostate cancer. J Endourol. 2000;14:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Beerlage HP, Thüroff S, Madersbacher S, Zlotta AR, Aus G, de Reijke TM, de la Rosette JJ. Current status of minimally invasive treatment options for localized prostate carcinoma. Eur Urol. 2000;37:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Ashush H, Rozenszajn LA, Blass M, Barda-Saad M, Azimov D, Radnay J, Zipori D, Rosenschein U. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res. 2000;60:1014-1020. [PubMed] |
| 4. | Abdollahi A, Domhan S, Jenne JW, Hallaj M, Dell'Aqua G, Mueckenthaler M, Richter A, Martin H, Debus J, Ansorge W. Apoptosis signals in lymphoblasts induced by focused ultrasound. FASEB J. 2004;18:1413-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Wang XJ, Yuan SI, Zhang J, Lu YR, Wang YP, Chen XH, Ning QZ, Fu YR, Liu BT, Zeng WF. A study of the inhibition effect of HIFU and its mechanism of action on the proliferation of human breast cancer cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:60-63. [PubMed] |
| 6. | Wang X, Yuan S, Wang C. A preliminary study of the anti-cancer effect of tanshinone on hepatic carcinoma and its mechanism of action in mice. Zhonghua ZhongLiu ZaZhi. 1996;18:412-414. [PubMed] |
| 7. | Beerlage HP, Thüroff S, Debruyne FM, Chaussy C, de la Rosette JJ. Transrectal high-intensity focused ultrasound using the Ablatherm device in the treatment of localized prostate carcinoma. Urology. 1999;54:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Chapelon JY, Ribault M, Vernier F, Souchon R, Gelet A. Treatment of localised prostate cancer with transrectal high intensity focused ultrasound. Eur J Ultrasound. 1999;9:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Bai J, Li F, Du Y, Wen S, Hu K, Xu G, Ma P, Yin N, Chen W. Study of a "biological focal region" of high-intensity focused ultrasound. Ultrasound Med Biol. 2003;29:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Yang R, Reilly CR, Rescorla FJ, Faught PR, Sanghvi NT, Fry FJ, Franklin TD, Lumeng L, Grosfeld JL. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg. 1991;126:1002-109; discussion 1002-109;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 116] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Cheng SQ, Zhou XD, Tang ZY, Yu Y, Bao SS, Qian DC. Iodized oil enhances the thermal effect of high-intensity focused ultrasound on ablating experimental liver cancer. J Cancer Res Clin Oncol. 1997;123:639-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Adams JB, Moore RG, Anderson JH, Strandberg JD, Marshall FF, Davoussi LR. High-intensity focused ultrasound ablation of rabbit kidney tumors. J Endourol. 1996;10:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Madersbacher S, Kratzik C, Susani M, Pedevilla M, Marberger M. Transcutaneous high-intensity focused ultrasound and irradiation: an organ-preserving treatment of cancer in a solitary testis. Eur Urol. 1998;33:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Van Leenders GJ, Beerlage HP, Ruijter ET, de la Rosette JJ, van de Kaa CA. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Prat F, Centarti M, Sibille A, Abou el Fadil FA, Henry L, Chapelon JY, Cathignol D. Extracorporeal high-intensity focused ultrasound for VX2 liver tumors in the rabbit. Hepatology. 1995;21:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Sibille A, Prat F, Chapelon JY, Abou el Fadil F, Henry L, Theillère Y, Ponchon T, Cathignol D. Extracorporeal ablation of liver tissue by high-intensity focused ultrasound. Oncology. 1993;50:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yang R, Sanghvi NT, Rescorla FJ, Kopecky KK, Grosfeld JL. Liver cancer ablation with extracorporeal high-intensity focused ultrasound. Eur Urol. 1993;23 Suppl 1:17-22. [PubMed] |
| 18. | Sibille A, Prat F, Chapelon JY, abou el Fadil F, Henry L, Theilliere Y, Ponchon T, Cathignol D. Characterization of extracorporeal ablation of normal and tumor-bearing liver tissue by high intensity focused ultrasound. Ultrasound Med Biol. 1993;19:803-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Arefiev A, Prat F, Chapelon JY, Tavakkoli J, Cathignol D. Ultrasound-induced tissue ablation: studies on isolated, perfused porcine liver. Ultrasound Med Biol. 1998;24:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Science Editor Wang XL and Guo SY Language Editor Elsevier HK