Published online Jul 28, 2005. doi: 10.3748/wjg.v11.i28.4305
Revised: March 3, 2005
Accepted: March 10, 2005
Published online: July 28, 2005
AIM: To investigate the relationship between the superoxide dismutase (SOD), malondialdehyde (MDA) metabolic changes and the gastric carcinogenesis.
METHODS: The SOD activity and MDA content were measured in the gastric tissues from the focus center, peripheral and far-end areas of gastric carcinoma (n = 52) and gastric ulcer (n = 10). All the tissues were subjected to routine histological examinations and classifications.
RESULTS: The SOD activity was greatly reduced but the MDA content was markedly increased in the center areas of the non-mucous gastric carcinoma (non-MGC); and the poorly differentiated gastric carcinoma varied. The SOD activity was gradually decreased and the MDA content was gradually increased in the tissues from the focus far-end, peripheral to center areas of non-MGC. Both of the SOD activity and the MDA content were significantly declined and were respectively at same low level in the tissues from the focus center, peripheral, and far-end area with the mucous gastric carcinoma (MGC). In contrast to the gastric ulcer and grade I or II of non-MGC, the same level of the SOD activity and the MDA content were found in the focus center areas. Between non-MGC (groups A-D) and gastric ulcer (group F), the differences of SOD activity and MDA content were very noticeable in the gastric tissues from the focus peripheral and far-end areas, in which the SOD activity showed noticeable increase and the MDA content showed noticeable decrease in the gastric ulcer.
CONCLUSION: The active free radical reaction in the gastric tissues can induce the carcinogenesis of non-MGC. The utmost low ability of antioxidation in the gastric tissues can induce the carcinogenesis of MGC. The metabolic change of the free radicals centralized mostly in the center of ulcerated lesions only, which suggested the ability of antioxidation was declined only in these lesions. However, the metabolism of free radicals varied significantly and the ability of antioxidation declined not only in the local focus area but also in the abroad gastric tissues with gastric carcinoma.
- Citation: Wang SH, Wang YZ, Zhang KY, Shen JH, Zhou HQ, Qiu XY. Effect of superoxide dismutase and malondialdehyde metabolic changes on carcinogenesis of gastric carcinoma. World J Gastroenterol 2005; 11(28): 4305-4310
- URL: https://www.wjgnet.com/1007-9327/full/v11/i28/4305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i28.4305
Free radical is the middle product during the phase of the biochemical metabolism in the body[1], and it is the intense toxicant because of being very active in the biochemical nature and oxidizing ability[2-5]. Although recent work has shown that free radical is related to the causing and development of many digestive system diseases[6-8], it did not distinct the relationship between the free radical and the gastric carcinogenesis[9,10]. In the investigation, it was researched that metabolic changes of the free radical play a role in the gastric carcinogenesis, development of gastric carcinoma by investigating the metabolism condition and regularity of the free radicals in the fresh samples of the gastric carcinoma tissues.
From 1998 to 2002, 52 cases of gastric carcinoma and 10 cases of gastric ulcer were obtained from the Central Hospital of Shantou City. All fresh stomach samples were surgically removed from these patients and regular routine histological examination and classification were made. The patients with gastric carcinoma were 11 women and 41 men. The mean age was 59.83 years, ranging from 34 to 76 years. The patients with gastric ulcer were 1 woman and 9 men and the mean age was 53.30 years, ranging from 19 to 69 years.
Stomach samples were obtained, the tissues at the center, peripheral (2 cm away from focus lesion) and far-end areas (8-10 cm away from focus lesion) sampled respectively. The fresh tissues were made into 10% tissue homogenate for measurement of the superoxide dismutase (SOD) activity and malondialdehyde (MDA) content. The SOD activity was examined with xanthine oxidase method and the MDA content was examined with sulfur barbituric acid method[11]. SOD and MDA detection kits were purchased from Nanjing Jiancheng Bioengineering Institute.
All data were disposed by SPSS9.0 statistical software. Statistical comparisons between interior groups were analyzed with ANOVA followed by Student’s t-test. Statistical comparisons between different groups were analyzed with one-way ANOVA followed by q-test. Experimental results were finally expressed as mean±SD. P value less than 0.05 was considered statistically significant.
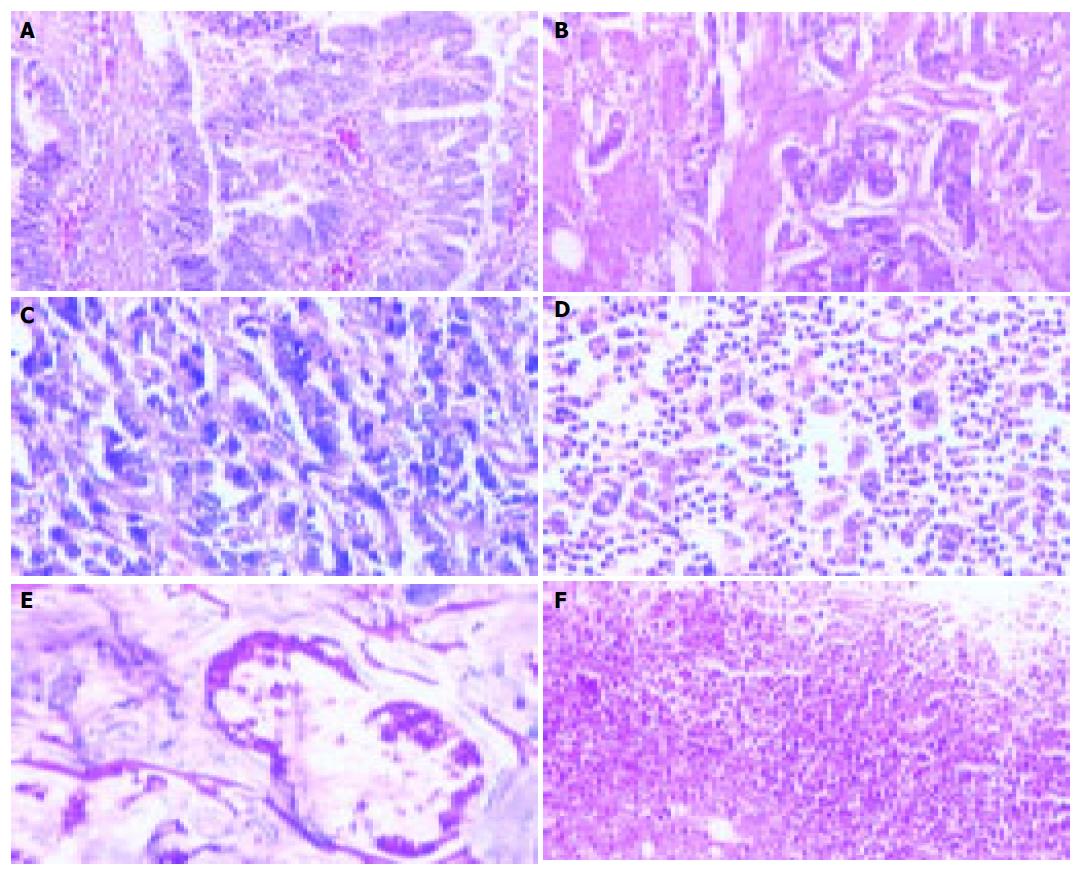
Fifty-two patients with gastric carcinoma were confirmed by histological examination. Among the 52 cases, 44 were non-mucous gastric carcinoma (non-MGC) including papillary, tubular, poorly differentiated, undifferentiated carcinoma, and were graded into four groups: grade I, 12 cases (group A, Figure 1A); grade II, 15 cases (group B, Figure 1B); grade III, 11 cases (group C, Figure 1C); grade IV, 6 cases (group D, Figure 1D). Eight cases were mucous gastric carcinoma (MGC) including mucinous adenocarcinoma and signet-ring cell carcinoma (group E, Figure 1E). Another 10 patients who were confirmed had gastric ulcer (group F, Figure 1F).
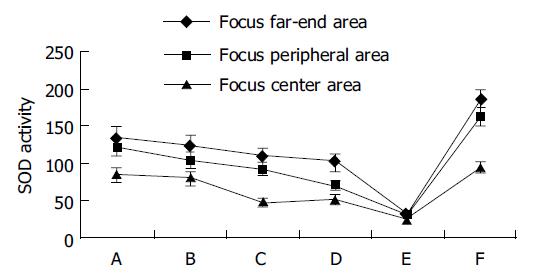
The SOD activity was declined gradually in the tissues from the focus far-end, peripheral, to center areas in non-MGC (groups A-D). The SOD activity in the focus center areas was the lowest in the measured gastric tissue. SOD activity reduction was correlated with the grade and differentiation of gastric carcinoma in the focus center areas, in which the level of the SOD activity in groups A and B (grades I and II) was higher than that in groups C and D (grades III and IV). There was no significant difference of the SOD activity in the tissues from the focus far-end areas between groups A-D.
The level of SOD activity was extremely low in the focus center areas in MGC (group E). The level of SOD activity was noticeably low in the focus peripheral and far-end areas. Although reduction tendency was minimized, there were no significant differences of the SOD activity in the tissues from the focus far-end, peripheral to center areas; suggesting that the SOD activity in these areas was at the same low level.
The level of SOD activity was low in the focus center area with gastric ulcer (group F). Notable reduction tendency about SOD activity was shown in the tissues from the focus far-end, peripheral to center areas. The common metabolic characteristics were found in gastric ulcer (group F) and non-MGC (groups A-D). The SOD activity was gradually declined from the focus far-end, peripheral to center areas. The SOD activity was at the same level in the tissues from the focus center areas between gastric ulcer (group F) and non-MGC (grades I and II, groups A and B). Importantly, the SOD activity was noticeably higher in the tissues from the focus peripheral and far-end areas in the gastric ulcer (group F) than non-MGC (groups A-D). To compare the gastric ulcer (group F) and the MGC (group E), the level of SOD activity was noticeably higher in all the tissues in the former than the later, and the SOD activity showed a gradual reduction tendency in the former, same low level in the later from the focus far-end, peripheral to center areas (Table 1 and Figure 2).
| Group | n | Focus center area (1) | Focus peripheral area (2) | Focus far-end area (3) | |
| Non-MGC | |||||
| I (A) | 12 | 85.88±8.97 | 122.79±12.08 | 135.33±14.74 | |
| II (B) | 15 | 81.26±8.68 | 104.73±11.24 | 123.13±14.46 | |
| III (C) | 11 | 49.01±4.97 | 93.35±8.32 | 109.59±11.45 | |
| IV (D) | 6 | 52.05±5.13 | 70.71±6.01 | 102.25±10.34 | |
| MGC (E) | 8 | 23.64±2.31 | 31.34±3.79 | 33.47±4.78 | |
| Gastric ulcer (F) | 10 | 95.63±8.01 | 162.88±12.26 | 186.22±11.74 | |
| P: (Statistical comparisons interior group) | |||||
| ①A1:A2<0.05 | A1:A3<0.01 | A2:A3>0.05 | ②B1:B2>0.05 | B1:B3<0.05 | B2:B3>0.05 |
| ③C1:C2<0.01 | C1:C3<0.01 | C2:C3>0.05 | ④D1:D2<0.05 | D1:D3<0.01 | D2:D3<0.05 |
| ⑤E1:E2>0.05 | E1:E3>0.05 | E2:E3>0.05 | ⑥F1:F2<0.01 | F1:F3<0.01 | F2:F3>0.05 |
| P: (Statistical comparisons between different groups) | |||||
| ①A1:B1>0.05 | A1:C1<0.01 | A1:D1<0.05 | A1:E1<0.01 | A1:F1>0.05 | B1:C1<0.01 |
| B1:D1<0.05 | B1:E1<0.05 | B1:F1>0.05 | C1:D1>0.05 | C1:E1<0.05 | C1:F1<0.01 |
| D1:E1<0.05 | D1:F1<0.01 | E1:F1<0.01 | |||
| ②A2:B2>0.05 | A2:C2<0.05 | A2:D2<0.01 | A2:E2<0.01 | A2:F2<0.05 | B2:C2<0.05 |
| B2:D2:<0.05 | B2:E2<0.01 | B2:F2<0.01 | C2:D2<0.05 | C2:E2<0.01 | C2:F2<0.01 |
| D2:E2<0.05 | D2:F2<0.01 | E2:F2<0.01 | |||
| ③A3:B3>0.05 | A3:C3>0.05 | A3:D3>0.05 | A3:E3<0.01 | A3:F3<0.01 | B3:C3>0.05 |
| B3:D3>0.05 | B3:E3<0.01 | B3:F3<0.01 | C3:D3>0.05 | C3:E3<0.01 | C3:F3<0.01 |
| D3:E3<0.01 | D3:F3<0.01 | E3:F3<0.01 | |||
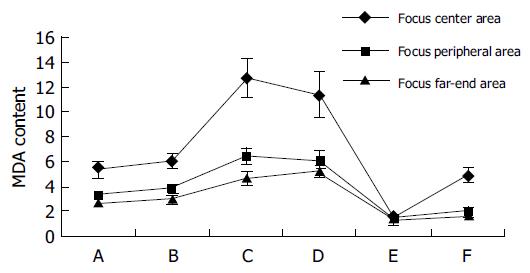
The MDA content increased gradually in the tissues from the focus far-end, peripheral to center areas in non-MGC (groups A-D). The MDA content in the focus center areas was the highest in the measured gastric tissue. The level of MDA content correlated with the grade and differentiation of gastric carcinoma in the tissues from the center areas. The study showed that the level of the MDA content in the groups A and B (grades I and II) was lower than groups C and D (grades III and IV). There were significant differences of the MDA content in the tissues from far-end areas, in which the level of MDA content was noticeably lower in the groups A and B than the groups C and D.
The level of MDA content was extremely low in the center, peripheral, and far-end areas in MGC (group E). There was no significant difference of the MDA content; it was at the same low level, although a minimum gradual increase tendency was found in the tissues from the focus far-end, peripheral to center areas.
The level of MDA content was noticeably high in the tissues from the focus center areas in the gastric ulcer (group F). The gradual increase tendency also showed in the tissues from the focus far-end, peripheral to center areas. The common metabolic characteristics were that the MDA content showed a gradual increase tendency in the tissues from the focus far-end, peripheral to center areas between gastric ulcer (group F) and non-MGC (groups A-D). And both in the group F and groups A and B, the MDA content was at the same level in the tissues from the focus center areas. However, when the gastric ulcer (group F) compared to the non-MGC (groups A-D), there was a significant difference: the MDA content declined markedly and varied in the tissues from the focus peripheral and far-end areas. The MDA content was noticeably higher in the tissues from the focus center areas in the gastric ulcer (group F) than the MGC (group E). But, the MDA content was at the same level in the tissues from the peripheral and focus far-end. And in the gastric ulcer (group F) compared to the MGC (group E), it showed a gradual increase tendency in the former, the later was at same low level in the tissues from the focus far-end, peripheral to center areas (Table 2 and Figure 3).
| Group | n | Focus center area (1) | Focus peripheral area (2) | Focus far-end area (3) | |
| Non-MGC | |||||
| I (A) | 12 | 5.37±0.61 | 3.28±0.23 | 2.56±0.18 | |
| II (B) | 15 | 6.07±0.62 | 3.85±0.31 | 2.96±0.25 | |
| III (C) | 11 | 12.75±1.52 | 6.46±0.65 | 4.65±0.52 | |
| IV (D) | 6 | 11.41±1.91 | 6.10±0.71 | 5.20±0.45 | |
| MGC (E) | 8 | 1.55±0.23 | 1.43±0.18 | 1.12±0.12 | |
| Gastric ulcer (F) | 10 | 4.89±0.56 | 2.06±0.22 | 1.68±0.16 | |
| P: (Statistical comparisons interior group) | |||||
| ①A1:A2<0.01 | A1:A3<0.01 | A2:A3<0.05 | ②B1:B2<0.01 | B1:B3<0.01 | B2:B3<0.01 |
| ③C1:C2<0.01 | C1:C3<0.01 | C2:C3<0.05 | ④D1:D2<0.05 | D1:D3<0.05 | D2:D3>0.05 |
| ⑤E1:E2>0.05 | E1:E3>0.05 | E2:E3>0.05 | ⑥F1:F2<0.01 | F1:F3<0.01 | F2:F3>0.05 |
| P: (Statistical comparisons between different groups) | |||||
| ①A1:B1>0.05 | A1:C1<0.01 | A1:D1<0.01 | A1:E1<0.01 | A1:F1>0.05 | B1:C1<0.01 |
| B1:D1<0.05 | B1:E1<0.01 | B1:F1>0.05 | C1:D1>0.05 | C1:E1<0.01 | C1:F1<0.01 |
| D1:E1<0.01 | D1:F1<0.01 | E1:F1<0.05 | |||
| ②A2:B2>0.05 | A2:C2<0.01 | A2:D2<0.01 | A2:E2<0.01 | A2:F2<0.05 | B2:C2<0.01 |
| B2:D2<0.01 | B2:E2<0.01 | B2:F2<0.01 | C2:D2>0.05 | C2:E2<0.01 | C2:F2<0.01 |
| D2:E2<0.01 | D2:F2<0.01 | E2:F2>0.05 | |||
| ③A3:B3>0.05 | A3:C3<0.01 | A3:D3<0.01 | A3:E3<0.01 | A3:F3<0.05 | B3:C3<0.01 |
| B3:D3>0.01 | B3:E3<0.01 | B3:F3<0.01 | C3:D3>0.05 | C3:E3<0.01 | C3:F3<0.01 |
| D3:E3<0.01 | D3:F3<0.01 | E3:F3>0.05 | |||
SOD is the most important substance that eliminated free radical system in the cell; it makes superoxide anion free radical (O2–), dismutates into H2O2 and protects the cells from the focus damage by cleaning up O2–. The level of SOD activity represents the intracellular antioxidation ability[8,10,12]. MDA is the main metabolite in the lipid peroxidation reaction. The free radicals affect on the membrane structures of the cells, including the membranes of the cell, mitochondrion, lysosome, endoplasmic reticulum, etc., then injury of the cells. The level of MDA content represents the extent of the intracellular lipid peroxidation reaction[13-15]. The SOD activity and the MDA content are the main index of the metabolic condition of the free radicals. We acquired the new knowledge that the free radicals play a key role in the mechanism of the gastric carcinogenesis from our experimental data.
The gastric mucosa from the focus far-end, peripheral to center area in non-MGC showed a pathological change from normal, atypical hyperplasia to carcinoma, by morphological observation. The SOD activity also showed a gradual reduction and the MDA content a gradual increase in gastric tissues from the focus far-end, peripheral to center areas. These results showed that the intracellular antioxidation ability in the gastric tissue was gradually reduced and the free radical reaction was gradually active, resulting in the injury of cells by the lipid peroxidation reaction, which stimulated the pathological changes from normal gastric mucous membrane to carcinoma[16-18].
The results further showed that the lower the differentiation degree of the gastric carcinoma, the more prominent the change of the free radicals metabolism. The SOD activity was lower and the MDA content higher in the groups C and D (gastric carcinoma grades III and IV) than the groups A and B (gastric carcinoma grades I and II). These results suggest two possible mechanisms: the first one was that the active free radical reaction severely damages the gastric mucous membrane cells, resulting in malignant change in the gastric mucous membrane from the normal cell to poorly differentiated and undifferentiated carcinomas. The second was that the carcinoma cells with poorly differentiated, undifferentiated need to produce an amount of the active free radicals for cells blooming division and breeding for themselves[15,19].
Interestingly, our results showed that there was no significant difference of the SOD activity in the far-end area between groups A, B, C, and D. However, the differences of the MDA content were very noticeable, i.e., the MDA content of the groups C and D (gastric carcinoma grades III and IV) were very much higher than groups A and B (gastric carcinoma grades I and II), indicated that abnormal lipid peroxidation reaction of the gastric tissue in far-end areas in groups C and D was not regulated by the SOD activity, and the reduction of the ability of the antioxidation was correlated with the widespread infiltration of the gastric carcinoma tissue (gastric carcinoma grades III and IV).
The tendency changes of the SOD activity and the MDA content of gastric tissues in non-MGC (groups A-D) were consistent with that in gastric ulcer (group F) from the focus center, peripheral to far-end areas. Between groups A, B and group F, the same level of the SOD activity and the MDA content were found in the gastric tissues from the focus center areas. Importantly, between the non-MGC (groups A-D) and the gastric ulcer (group F), the differences of the SOD activity and the MDA content were highly noticeable in the gastric tissues from the focus peripheral and far-end areas, which the SOD activity showed noticeable increase and the MDA content showed noticeable decrease in the gastric ulcer. It was suggested that there was high ability of the antioxidation for protecting itself against damage by the free radicals and could balance free radical metabolism in the gastric tissue from the focus peripheral and far-end areas in the gastric ulcer (group F). Meantime, it was suggested that the changes of the free radicals metabolism centralized mostly in the center area of gastric ulcer, where the ability of the antioxidation was reduced only in the local focus. Compared to the gastric ulcer (group F), the variation of the SOD activity and the MDA content were very small in the peripheral area and far-end area in the non-MGC (groups A-D), suggested that the ability of the antioxidation was reduced not only in the local focus area but also in the abroad gastric tissues. This imbalance of the free radical metabolism occurs in whole gastric tissue, resulting in the reduction of the protection itself of the gastric tissue and inducing the gastric carcinogenesis, i.e., the arising of non-MGC[20,21].
The mucinous adenocarcinoma and the signet-ring cell carcinoma arising from the gastric mucosa possess large quantity of mucus, inside or/and around the cancer cells. And because of that characteristic nature, the cancer cells have strong ability of infiltration and widespread distribution in the gastric tissue. There were obvious differences of the SOD activity and the MDA content in gastric mucinous adenocarcinoma and signet-ring cell carcinoma, and classified as a special group (group E), compared to other type gastric carcinoma. The obvious reduction and the same low level of the SOD activity and the MDA content were found in the tissues of the focus center, peripheral and far-end areas in the MGC, where the free radical metabolism changed obviously. The data in previous experiments showed an inverse relationship between the SOD activity and the MDA content in the tissues[13,15]. The low SOD activity induced the reduction of the antioxidation ability and the increase of the lipid peroxidation reaction, the metabolic product, MDA, in the tissues. On the contrary, the increase of the SOD activity inhibited the reaction of the lipid peroxidation, resulting in the reduction of the metabolic product, MDA, in the tissues. But in this study, the SOD activity decreased greatly in the gastric tissue in the MGC, and the MDA content as well. Interestingly, the SOD activity and the MDA content decreased markedly to the same low level in the tissues wherever focus center, peripheral and far-end areas in the MGC. This condition of the free radicals metabolism maybe related with the special pathological changes, where there was an abundance of mucus in the tissues. The SOD is the most important substance to eliminate free radical system in cells and tissues and the level of SOD activity represents the intracellular antioxidation ability[12,22]. The utmost low level of the SOD activity represents the utmost low level of the intracellular antioxidation ability in gastric tissue with the MGC. It was considered accordingly that the antioxidation ability was the base and precondition in maintaining the normal physiological function, differentiation, division, multiplication of the cells[20,23]. The loss of the antioxidation ability may contribute to make the normal cells become carcinoma cells[9,24-26] and the gastric tissues cannot resist the infiltration of the cancer cells, resulting in cancer cells spreading extensively in stomach wall.
Compared to gastric ulcer, there was a very low level of the SOD activity in gastric tissues of center, peripheral and far-end areas with the MGC and the low antioxidation ability and the low defense function by themselves. The MDA content, in the tissues from the focus peripheral and far-end areas with the MGC and the ulcer, both were at the same low level. But the SOD activity was increased greatly in the tissues from the focus peripheral and far-end area with gastric ulcer (group F). It suggested that there was still a very strong antioxidation and protecting ability in the gastric tissues from the peripheral and far-end areas with the gastric ulcer (group F). It also confirmed that the extreme low antioxidation ability and the low SOD activity in the gastric tissues in the MGC (group E) were related to the causing and development of the MGC, and can induce the formation of the MGC. On the other hand, the very low level of the MDA suggests the lipid peroxidation reaction was inactive, which indicated that the arising of the MGC was not related with the lipid peroxidation reaction to damage of the tissues.
| 1. | Khanzode SS, Khanzode SD, Dakhale GN. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Lett. 2003;195:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Janssen AM, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res. 2000;6:3183-3192. [PubMed] |
| 3. | Fujisawa S, Atsumi T, Ishihara M, Kadoma Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004;24:563-569. [PubMed] |
| 4. | Sander CS, Chang H, Hamm F, Elsner P, Thiele JJ. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 5. | Sanders PM, Tisdale MJ. Role of lipid-mobilising factor (LMF) in protecting tumour cells from oxidative damage. Br J Cancer. 2004;90:1274-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Kim JJ, Chae SW, Hur GC, Cho SJ, Kim MK, Choi J, Nam SY, Kim WH, Yang HK, Lee BL. Manganese superoxide dismutase expression correlates with a poor prognosis in gastric cancer. Pathobiology 2002-. 2003;70:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Korenaga D, Yasuda M, Honda M, Nozoe T, Inutsuka S. MnSOD expression within tumor cells is closely related to mode of invasion in human gastric cancer. Oncol Rep. 2003;10:27-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Yasuda M, Takesue F, Inutsuka S, Honda M, Nozoe T, Korenaga D. Prognostic significance of serum superoxide dismutase activity in patients with gastric cancer. Gastric Cancer. 2002;5:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Toh Y, Kuninaka S, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Mori M, Sugimachi K. Overexpression of manganese superoxide dismutase mRNA may correlate with aggressiveness in gastric and colorectal adenocarcinomas. Int J Oncol. 2000;17:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Izutani R, Asano S, Imano M, Kuroda D, Kato M, Ohyanagi H. Expression of manganese superoxide dismutase in esophageal and gastric cancers. J Gastroenterol. 1998;33:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Pang ZJ, Zhou M, Chen Y. The research method of free radicals medical science. 1st. Beijing: PEOPLE HYGIENE Pub 2000; 64 –66. |
| 12. | Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Arivazhagan S, Kavitha K, Nagini S. Erythrocyte lipid peroxidation and antioxidants in gastric cancer patients. Cell Biochem Funct. 1997;15:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Lee IS, Nishikawa A. Polyozellus multiplex, a Korean wild mushroom, as a potent chemopreventive agent against stomach cancer. Life Sci. 2003;73:3225-3234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Skrzydlewska E, Kozuszko B, Sulkowska M, Bogdan Z, Kozlowski M, Snarska J, Puchalski Z, Sulkowski S, Skrzydlewski Z. Antioxidant potential in esophageal, stomach and colorectal cancers. Hepatogastroenterology. 2003;50:126-131. [PubMed] |
| 17. | Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004;157:327-349. [PubMed] |
| 18. | Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;157:301-310. [PubMed] |
| 19. | Policastro L, Molinari B, Larcher F, Blanco P, Podhajcer OL, Costa CS, Rojas P, Durán H. Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol Carcinog. 2004;39:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Eapen CE, Madesh M, Balasubramanian KA, Pulimood A, Mathan M, Ramakrishna BS. Mucosal mitochondrial function and antioxidant defences in patients with gastric carcinoma. Scand J Gastroenterol. 1998;33:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Zavros Y, Kao JY, Merchant JL. Inflammation and cancer III. Somatostatin and the innate immune system. Am J Physiol Gastrointest Liver Physiol. 2004;286:G698-G701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Izutani R, Kato M, Asano S, Imano M, Ohyanagi H. Expression of manganese superoxide disumutase influences chemosensitivity in esophageal and gastric cancers. Cancer Detect Prev. 2002;26:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Zheng QS, Sun XL, Wang CH. Redifferentiation of human gastric cancer cells induced by ascorbic acid and sodium selenite. Biomed Environ Sci. 2002;15:223-232. [PubMed] |
| 24. | Magálová T, Bella V, Brtková A, Beno I, Kudlácková M, Volkovová K. Copper, zinc and superoxide dismutase in precancerous, benign diseases and gastric, colorectal and breast cancer. Neoplasma. 1999;46:100-104. [PubMed] |
| 25. | Shimoyama S, Aoki F, Kawahara M, Yahagi N, Motoi T, Kuramoto S, Kaminishi M. Early gastric cancer development in a familial adenomatous polyposis patient. Dig Dis Sci. 2004;49:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol. 2004;286:G515-G520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK