Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4277
Revised: January 6, 2005
Accepted: January 12, 2005
Published online: July 21, 2005
AIM: To investigate the potential role of nuclear factor kappa-B (NF-κB) activation on the reactive oxygen species in rat acute necrotizing pancreatitis (ANP) and to assess the effect of pyrrolidine dithiocarbamate (PDTC, an inhibitor of NF-κB).
METHODS: Rat ANP model was established by retrograde injection of 5% sodium taurocholate into biliopancreatic duct. Rats were randomly assigned to three groups (10 rats each): Control group, ANP group and PDTC group. At the 6th h of the model, the changes of the serum amylase, nitric oxide (NO), malondialdehyde (MDA), superoxide dismutase (SOD) and pancreatic morphological damage were observed. The expressions of inducible nitric oxide (iNOS) were observed by SP immunohistochemistry. And the expressions of NF-κB p65 subunit mRNA were observed by hybridization in situ.
RESULTS: Serum amylase and NO level decreased signifi-cantly in ANP group as compared with PDTC administrated group [(7 170.40 ± 1 308.63) U/L vs (4 074.10 ± 1 719.78) U/L, P < 0.05], [(76.95 ± 9.04) mol/L vs (65.18 ± 9.02) mol/L, P < 0.05] respectively. MDA in both ANP and PDTC group rose significantly over that in control group [(9.88 ± 1.52) nmol/L, (8.60 ± 1.41) nmol/L, vs (6.04 ± 1.78) nmol/L, P < 0.05], while there was no significant difference between them. SOD levels in both ANP and PDTC group underwent a significant decrease as compared with that in control [(3 214.59 ± 297.74) NU/mL, (3 260.62 ± 229.44) NU/mL, vs (3 977.80 ± 309.09) NU/mL, P < 0.05], but there was no significant difference between them. Though they were still higher than those in Control group, pancreas destruction was slighter in PDTC group, iNOS expression and NF-κB p65 subunit mRNA expression were lower in PDTC group as compared with ANP group.
CONCLUSION: We conclude that correlation among NF-κB activation, serum amylase, reactive oxygen species level and tissue damage suggests a key role of NF-κB in the pathogenesis of ANP. Inhibition of NF-κB activation may reverse the pancreatic damage of rat ANP and the production of reactive oxygen species.
- Citation: Long J, Song N, Liu XP, Guo KJ, Guo RX. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol 2005; 11(27): 4277-4280
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4277
Acute pancreatitis is clinically classified into mild and severe forms. Mild or edematous acute pancreatitis is a self-limiting disease with a low complication and mortality rate. However, ANP has an unacceptably high morbidity and mortality rate. Multiple therapeutic modalities have been suggested for acute pancreatitis, but none has been unambiguously proven to be effective yet. The major problem is that the pathophysiology of the disease is not fully understood[1,2].
Oxygen free radicals are molecules produced continuously in cells by several mechanisms. The generation of oxygen free radicals is physiologic. In most circumstances, oxygen free radicals are neutralized immediately by enzymatic scavengers. But when formation of oxygen free radicals overwhelms radical neutralization in cells, oxidative stress occurs. As they are very reactive, they react well with all biological substances such as proteins, polysaccharides, and nucleic acids, resulting in tissue injury. It has been suggested that oxygen free radicals are responsible for a wide variety of diseases or conditions, for they play an important role in the pathogenesis of pancreatitis in some experimental models, and they are involved in the initiation of pancreatitis. Also, it was reported that oxygen free radicals acted as important mediators of tissue damage in experimental acute pancreatitis[3,4].
Nuclear factor kappa-B (NF-κB) is a sequence-specific transcription factor known to be involved in inflammatory and immune responses. It plays an important role in physiologic and pathologic conditions as an inducible nuclear factor. NF-κB is able to mediate a variety of inflammatory mediators involved in acute pancreatitis, including cytokines and adhesion molecules, as well as specific inducible isoform of nitric oxide synthase enzymes. Recent experimental studies appeared to have shed some light on the intracellular signaling pathway in the inflammatory cascade in acute pancreatitis[5-7]. Hence, the role of NF-κB in acute pancreatitis has attracted more and more attention.
Therefore, this study was conducted to evaluate the role of NF-κB in experimental model of rat ANP and to analyze the role of NF-κB activation on nitric oxide (NO) and other reactive oxygen species in the pathogenesis of ANP.
We randomized 30 male Wistar rats (weighing 250-300 g) to three groups, Control group, ANP group, and PDTC group. After having fasted for 24 h before the experiment, and allowed only drinking water freely, all rats were intraperitoneally infused with 2.5% pentobarbital sodium. When the abdominal cavity was opened through the median incision, the common bile duct and the pancreatic duct were found. After intubation from the end of pancreatic duct, the pancreatic duct was shut both at the duodenal ampulla and near the hepatic hilum transiently to prevent regurgitation of the infusion into the liver or duodenum. The ANP and PDTC group were induced by slow and even infusion of 5% sodium taurocholate (Sigma, St. Louis, MO, USA) into the pancreatic duct. The Control group received infusion of saline of the same amount instead of sodium taurocholate. In addition to sodium taurocholate, PDTC group received intravenous infusion of PDTC (Sigma, St. Louis, MO, USA) 10 mg/kg, while Control and ANP group received the same amount of saline instead.
At the 6th h of the model, blood was collected from the abdominal aorta of rats, and the pancreas was removed according to the following measurement: (1) Serum amylase detected by HITACHI 7170 automatic biochemical analyzer; (2) Ratio of NO2- to NO3- determined by copper-zinc-cadmium reductive chromatometry, which reflects NO level; (3) Malondialdehyde (MDA) detected by TBA chromatometry; (4) Superoxide dismutase (SOD) detected by hydroxylamine chromatometry; (5) Morphological damage to pancreas observed microscopically after fixation in 10% neutral formaldehyde solution and HE stain, morphological alterations were graded using a scale of the degree of inflammation, necrosis and edema; (6) Expressions of iNOS in pancreatic tissue detected by iNOS immunohistochemical kits (Beijing Zhongshan Biotechnology Co., Ltd. Beijing, China) iNOS antibody was diluted 50 fold, tested with SP method, and cells stained with brown-yellow granules were considered to be positive; (7) Expressions of NF-κB p65 subunit mRNA in pancreatic tissue: After fixation in 4% citromint and 1% DEPC, expression of NF-κB p65 subunit mRNA in situ kits (Wuhan Boster Bioengineering Co., Ltd. Wuhan, Hubei, China) Plasma and nucleus of pancreatic acinar stained with brown-yellow granules were considered to be positive. All immunohistochemistry or in situ images were analyzed and processed using MetaMorph v4.6 software (Universal Imaging Corp.). The intensity of expression was represented by average gray value, and the difference of average gray value reflected the difference of expression. Two pathologists assessed all of histopathologic sections in 20 fields/organ, and they were not aware as to which groups the sections belonged.
SASS 6.12 statistical analytic software was applied to process the data collected, and P < 0.05 was considered statistically significant.
After the above treatment, serum amylase in ANP group rose rapidly. Although it was significantly higher than that in control group (P < 0.05), serum amylase of PDTC group was still significantly lower than that in ANP group (P < 0.05). NO levels in both ANP and PDTC group increased. The former, however, was significantly higher than the latter (P < 0.05). MDA in both ANP and PDTC group rose significantly over that in control group (P < 0.05), while there was no significant difference between the two results themselves. SOD levels in both ANP and PDTC group underwent a significant decrease as compared with that in control group (P < 0.05), but there was no significant difference between either of them (Table 1).
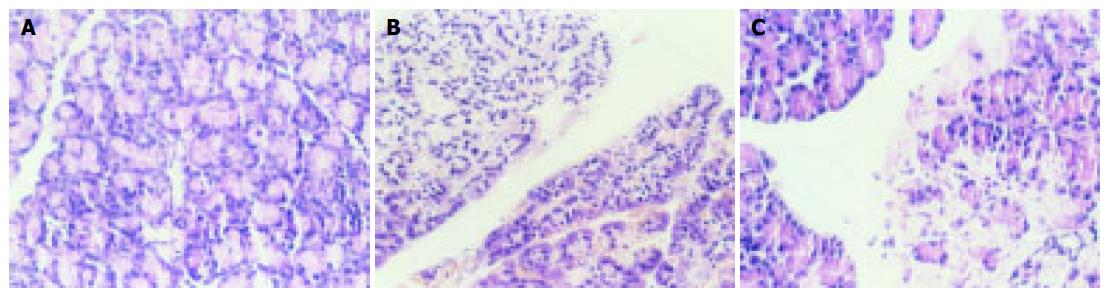
The only gross change in the abdominal cavity in control group was the mildly edematous pancreas. No structural damage was found microscopically in the pancreatic tissue, except for some local interstitial edema. In ANP group, bloody ascites, necrotic foci in pancreas, and fat necrosis in mesentery and omentum were found grossly. Interstitial lobular inflammatory infiltrations were observed in pancreas microscopically, as well as diffusive bleeding and piecemeal necrosis. In PDTC group, ascites and fat necrosis diminished notably compared with those in ANP group. Microscopically, slight bleeding, mild acinar degeneration and mild structure damage to lobules were observed together with declining inflammatory infiltration. The damage of ANP group is obviously grave than PDTC group [Histopathologic score: (5.76 ± 1.12) vs (4.00 ± 1.50), P < 0.05] (Figure 1).
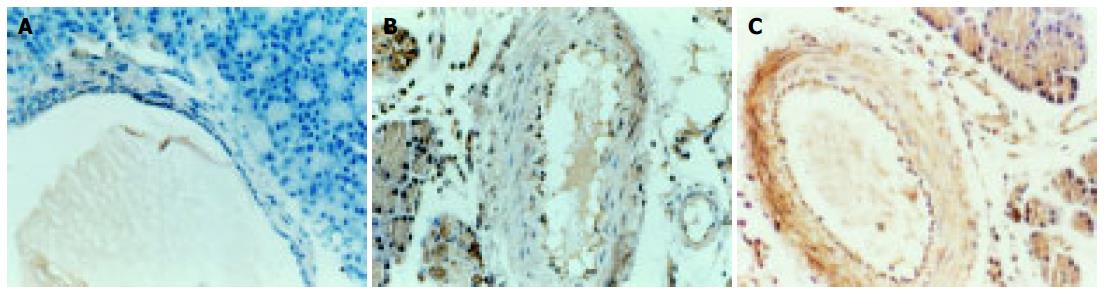
Expression of iNOS was negative in control group while positive in both ANP and PDTC group, mainly found in endotheliums and smooth muscle cells. The iNOS expression is significantly lower with PDTC group than ANP group [Average gray value: (80.43 ± 10.48) vs (64.26 ± 9.18), P < 0.05].
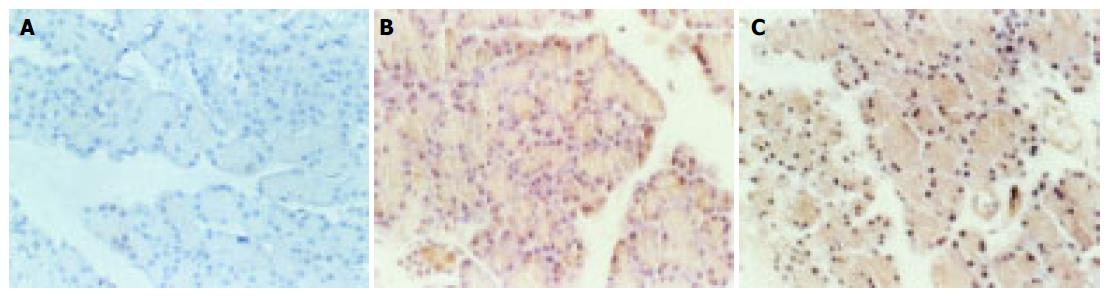
In control group, expression of NF-κB was negative in all acinar nuclei, and positive only in some plasma. In ANP group, however, the expression was positive in both nucleus and plasma of pancreatic acinar. And the expression in nucleus and plasma decreased significantly in PDTC group [Average gray value: (104.25 ± 19.08) vs (67.28 ± 8.95), P < 0.05].
One of the most severe complications of ANP is multiple system organ failure (MSOF) in early stage. The early systemic complication is the major cause of death in ANP, which leads to a mortality rate of 20%. Although it has been indicated that trypsin activation, auto-digestion of pancreas, cytokines, endotoxin, reactive oxygen species, and arachidnate[8-11] play an important role in the progression of MSOF in ANP, the mechanisms of the development of this disease remain obscure.
Like what happens in other inflammatory diseases, reactive oxygen species are generated in the early stage of ANP, and they play quite a vital role in the onset and development of ANP[11]. During ANP, activated neutrophiles are attached to endothelial cells, infiltrate into tissues, and produce large amount of reactive oxygen species and a kind of cytokines, which may cause severe damage to the pancreatic tissue. NO, in addition to potent function of vasodilation, can also inhibit the adherence, infiltration, and activation of white blood cells, and affect the production of reactive oxygen species[11,12]. NO is generated by two classes of nitric oxide synthase (NOS): One that is constitutive, Ca2+-dependent and physiologically activated (cNOS) and the other is inducible (iNOS). cNOS produces temperate amount of NO relieving ANP, whereas iNOS produces excess NO exacerbating the damage of ANP to the body[12].
Excessive production of NO causes vasodilatation and hypotension leading to organ hypoperfusion, edema, and organ dysfunction. Moreover, the reaction of NO with superoxide causes the formation of peroxynitrite, which is a powerful oxidant and cytotoxic agent and may play an important role in the cellular damage associated with the overproduction of NO. The spontaneous reaction of peroxynitrite with proteins makes the nitration of tyrosine residues to form nitrotyrosine, which is a specific nitration product of peroxynitrite and a marker for peroxynitrite-induced oxidative tissue damage. In this study, we found the concentration of SOD as an antioxidant decreased and that of MDA as the lipid peroxide increased, indicating the role of NO on the free radical reaction and oxidation response could intensify ANP.
NF-κB is a kind of pleiotropic regulative protein of transcription. Its activation takes part in the pathogenesis of ANP. Inhibition of the action can ameliorate the rat ANP[13,14]. NF-κB is also capable of regulating the expression of multiple inflammatory genes, such as tumor necrosis factor α (TNF-α), interleukin-6,8 (IL-6,8), and iNOS[15]. Over-expressions of these discussed genes, however, can cause damage to the pancreatic and extra-pancreatic tissues in ANP. NF-κB activation does relate to the reactive oxygen species in ANP.
Our study provides evidence that the injection of sodium taurocholate can cause ANP, with manifestation of the rise of serum amylase and NO, MDA level, damage to pancreas, inflammatory infiltration, and the decrease of SOD. Hybridization in situ and immunohistochemical results suggest that NF-κB is activated immediately at the onset of ANP, accompanied by high expression of iNOS. And there is otherwise no expression of NF-κB and iNOS under physiological conditions. The lower expression of NF-κB p65 subunit mRNA in PDTC group indicates that the administration of PDTC may inhibit the NF-κB activation. PDTC also leads to lower increase in serum amylase and slighter histological damage to pancreas. All these results are consistent with previous studies, substantiating that NF-κB activation is involved in the pathogenesis of ANP, and inhibition of the activation may reverse rat ANP[13,14]. Compared with those in ANP group, the NO and MDA level decreases, SOD level increases, and iNOS expression decreases. It is the increase of iNOS expression that aggravates ANP. Expression of iNOS is regulated by NF-κB activation and inhibition of the activation may reduce iNOS expression, thus relieve ANP. Correlation among NF-κB activation, NO, MDA, SOD level, tissue damage, and iNOS expression suggests a key role of NF-κB activation in the pathogenesis of ANP model. By inhibiting iNOS expression, NF-κB activation sets back over-production of reactive oxygen species like NO, therefore it reverses the damage of ANP to rats.
We may finally draw the conclusion from the above study that NF-κB activation in rat ANP may reduce over-production of reactive oxygen species, thus ameliorate the severity of ANP, all of which is achieved by inhibiting iNOS expression. The drug that can inhibit the activation of NF-κB may become a way of the therapy of ANP.
| 1. | Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 2. | Banks PA. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 1997;92:377-386. [PubMed] |
| 3. | Schoenberg MH, Birk D, Beger HG. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62:1306S-1314S. [PubMed] |
| 4. | Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 5. | Jaffray C, Yang J, Carter G, Mendez C, Norman J. Pancreatic elastase activates pulmonary nuclear factor kappa B and inhibitory kappa B, mimicking pancreatitis-associated adult respiratory distress syndrome. Surgery. 2000;128:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1157-G1162. [PubMed] |
| 7. | Algül H, Tando Y, Schneider G, Weidenbach H, Adler G, Schmid RM. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatology. 2002;2:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Karne S, Gorelick FS. Etiopathogenesis of acute pancreatitis. Surg Clin North Am. 1999;79:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Denham W, Norman J. The potential role of therapeutic cytokine manipulation in acute pancreatitis. Surg Clin North Am. 1999;79:767-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 514] [Article Influence: 18.4] [Reference Citation Analysis (2)] |
| 11. | Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736-2750. [PubMed] |
| 12. | Jaworek J, Jachimczak B, Tomaszewska R, Konturek PC, Pawlik WW, Sendur R, Hahn EG, Stachura J, Konturek SJ. Protective action of lipopolysaccharidesin rat caerulein-induced pancreatitis: role of nitric oxide. Digestion. 2000;62:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402-G1414. [PubMed] |
| 14. | Dunn JA, Li C, Ha T, Kao RL, Browder W. Therapeutic modification of nuclear factor kappa B binding activity and tumor necrosis factor-alpha gene expression during acute biliary pancreatitis. Am Surg. 1997;63:1036-143; discussion 1036-143;. [PubMed] |
| 15. | Abraham E. NF-kappaB activation. Crit Care Med. 2000;28:N100-N104. [PubMed] |
Science Editor Guo SY Language Editor Elsevier HK