Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4237
Revised: December 15, 2004
Accepted: December 20, 2004
Published online: July 21, 2005
AIM: We studied the effect of colchicine combined with radiation on the survival of human hepatocellular carcinoma (HCC) HA22T/VGH cells.
METHODS: Twenty-four hours after treatment with 0-8 ng/mL colchicine, HA22T/VGH cells were irradiated at various doses (0, 1, 2, 4, and 8 Gy). Colony assay was performed to assess the surviving cell fraction. Survival curves were fitted by using a linear-quadratic model to estimate the sensitizer enhancement ratio (SER). Flow cytometry was used for cell cycle analysis.
RESULTS: Colchicine at lower concentrations (1 and 2 ng/mL) had obvious synergy with radiation to inhibit HCC cell growth, whereas higher concentrations (4 and 8 ng/mL) had only additive effect to radiation. Pretreatment with 1 and 2 ng/mL colchicine for 24-h enhanced cell killing by radiation with SERs of 1.21 and 1.53, respectively. G2/M arrest was only observed with higher colchicine doses (8 and 16 ng/mL) after 24-h treatment; this effect was neither seen with lower doses (1, 2, and 4 ng/mL) nor with any dose after only 1 h of treatment.
CONCLUSION: Our results suggest that colchicine has potential as an adjunct to radiotherapy for HCC treatment. Lower doses of colchicine possess radiosensitizing effects via some mechanism other than G2/M arrest. Further study is necessary to elucidate the mechanism.
- Citation: Liu CY, Liao HF, Shih SC, Lin SC, Chang WH, Chu CH, Wang TE, Chen YJ. Colchicine sensitizes human hepatocellular carcinoma cells to damages caused by radiation. World J Gastroenterol 2005; 11(27): 4237-4240
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4237
Colchicine interferes with microtubule formation, thereby affecting mitosis and other microtubule-dependent functions[1]. It has been used in clinical practice for a long time, including for the treatment of acute gout[2], prophylaxis of recurrent gout[3], Behcet’s disease[4], chronic hepatitis B[5], and primary biliary cirrhosis[6]. It is a relatively safe and an effective drug when used with appropriate dosage[7].
Hepatocellular carcinoma (HCC) is a highly malignant tumor with poor prognosis and high mortality. It is a common malignancy in Asian countries. In China, the incidence has been increasing over the past two decades. The age-adjusted rate of death per 100 000/year was 20.37 in the 1990s. In the USA, the incidence of HCC has approximately doubled over the past three decades[8]. Aggressive treatment with a variety of modalities, including surgery, transarterial chemoembolization, percutaneous ethanol injection, radiofrequency ablation, microwave coagulation therapy, and laser-induced thermotherapy have all been used in an attempt to control this disease. However, the overall 5-year survival is still around 5%. Few tumors are resectable because of advanced stage or the presence of associated liver disease[9,10].
Radiation therapy (RT) has not played an important role in HCC treatment in the past because normal liver tissue has low tolerance to radiation. Radiation hepatitis usually develops with whole liver irradiation at or above 35 Gy, yet this dose level may not be sufficient to eradicate the tumor. However, small portions of the liver can be irradiated with 50-60 Gy without significant long-term morbidity. Recent advances in RT including three-dimensional conformal radiation therapy (3DRT) limits the exposure of normal liver tissue and allows delivery of higher RT doses (40-80 Gy)[11]. 3DRT has improved treatment outcome and reduced normal liver damage in unresectable HCC[12]. There is a dose-response relationship with 3-DRT for primary HCC, with only the radiation dose being a significant factor for predicting treatment response[13]. It is important to develop novel ways to improve the efficacy of RT, not only by physical technique but also by pharmacological agents. The development of radiosensitizers in order to reduce the required cytotoxic RT dose for HCC is especially important for this disease that arises in the midst of radiosensitive normal tissue.
Other studies have demonstrated that cells are most radiosensitive in the G2/M phase, whereas the most resistant are in late S phase[14]. Because colchicine arrests the cell cycle at the G2/M phase, we designed this study to assess its effect in combination with radiation on HA22T/VGH, a poorly differentiated HCC cell line[15,16].
Colchicine (C22H25NO6, molecular weight 399.4, purity 95%) was purchased from Sigma Co. (St. Louis, MO, USA). The powder was dissolved in distilled water to form an aqueous stock solution and diluted with PBS before use.
The human poorly differentiated HCC cell line HA22T/ VGH[15,16] was cultured in DMEM (GIBCO, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS, Hyclone, Logan, UT, USA), NaHCO3 (10 mmol/L) and HEPES (20 mmol/L) at 37°C in a humidified 50 mL/L CO2 incubator. For routine subculturing, the cells were grown to near confluence, collected by 0.25% trypsin, counted using the trypan blue exclusion test, and adjusted to an initial density of approximately 104 cells/mL.
In preliminary work, we determined the cytotoxic effect of different colchicine concentrations on HA22T/VGH HCC cells by using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay and found that concentrations up to 2 ng/mL were non-toxic. Based on these preliminary results, we used colchicine concentrations of 0-8 ng/mL to test for radiosensitization. HA22T/VGH HCC cells were treated with 0, 1, 2, 4, and 8 ng/mL colchicine for 24 h, and the colchicine was washed out before RT. RT with 6 MeV electron beam energy was delivered by a linear accelerator (Clinac 1800, Varian Associates, Inc., CA, USA; dose rate 2.4 Gy/min) at various doses (0, 1, 2, 4, and 8 Gy) in a single fraction. Full electron equilibrium was ensured for each fraction by a parallel plate PR-60C ionization chamber (CAPINTEL, Inc., Ramsey, NJ, USA). One hundred and fifty viable tumor cells were plated onto 35-mm six-well culture dishes and were allowed to grow in DMEM medium containing 10% heat-inactivated FCS and the various concentrations of colchicine at 37°C in a humidified 50 mL/L CO2 incubator for 24 h. Then the cells were treated with various radiation doses. Following radiation, a colony assay was performed.
After 10-14 d, the culture dishes were stained with 3% crystal violet and colonies (≥50 cells) were counted. The surviving fraction was calculated as mean colonies/cells inoculated. The mean plating efficiency for untreated HA22T/VGH HCC cells was 43%. Survival curves were fitted by using a linear-quadratic model[17]. The sensitizer enhancement ratio (SER) was calculated as the radiation dose needed for radiation alone divided by the dose needed for colchicine plus radiation at a surviving fraction of 37% (D0 in radiobiology).
Cells were treated with various doses of colchicine (0, 1, 2, 4, 8, and 16 ng/mL) for 1 or 24 h, then harvested and fixed at 4°C for 1 h with 70% ethanol. The cells were stained for 30 min with propidium iodide (PI) solution (PI, 0.5 mg/mL; RNAse, 0.1 mg/mL; Sigma Co.) from a CycleTEST plus DNA reagent kit (Becton Dickinson, Lincoln Park, NJ, USA). Analysis of DNA content was performed using a FACScalibur flow cytometer (Becton Dickinson). The data from 104 cells were collected and analyzed using ModFit software (Becton Dickinson).
Data were expressed as percentage and mean±SE.
We used Sigma Plot software (version 8.0, SPSS Inc., Chicago, IL, USA) to fit survival curves with a linear-quadratic model. One-way analysis of variance was used to compare the colony formation between different groups (SPSS, version 10.0, Chicago, IL, USA).
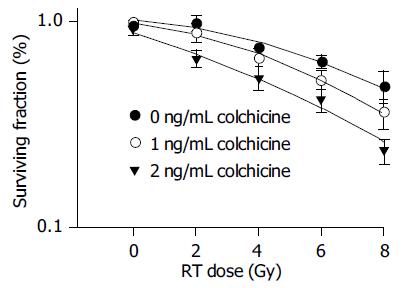
The results of colony assays of various doses colchicine combined with radiation are shown in Table 1. There was an obvious synergistic effect of low-dose colchicine (1 and 2 ng/mL) plus radiation. When the dose was increased to 4 and 8 ng/mL, only mild additive effects were noted.
| Colchicine (ng/mL) | 0 | 1 | 2 | 4 | 8 |
| RT 0 Gy | 81.0 ± 4.0 | 78.7 ± 1.0 | 72.8 ± 3.3 | 57.5 ± 4.0 | 29.2 ± 2.7 |
| 1 | 78.3 ± 4.1 | 70.5 ± 5.1 | 52.7 ± 4.5 | 43.3 ± 4.3 | 22.3 ± 3.0 |
| 2 | 59.8 ± 3.5 | 53.5 ± 4.2 | 42.3 ± 3.6 | 33.2 ± 2.5 | 12.8 ± 3.0 |
| 4 | 51.2 ± 3.0 | 41.5 ± 3.2 | 32.8 ± 3.2 | 27.0 ± 3.3 | 11.5 ± 2.7 |
| 8 | 38.2 ± 5.6 | 27.3 ± 3.5 | 18.5 ± 1.8 | 15.3 ± 2.4 | 6.7 ± 1.8 |
As the survival curves in Figure 1 demonstrate, low-dose colchicine sensitized HA22T/VGH HCC cells to radiation in a dose-dependent manner. The RT doses required for a surviving fraction of 37% (D0 in radiobiology) after pretreatment with 0, 1, and 2 ng/mL colchicine were 9.45, 7.80, and 6.16 Gy respectively. The calculated SERs of colchicine were 1.21 for 1 ng/mL and 1.53 for 2 ng/mL.
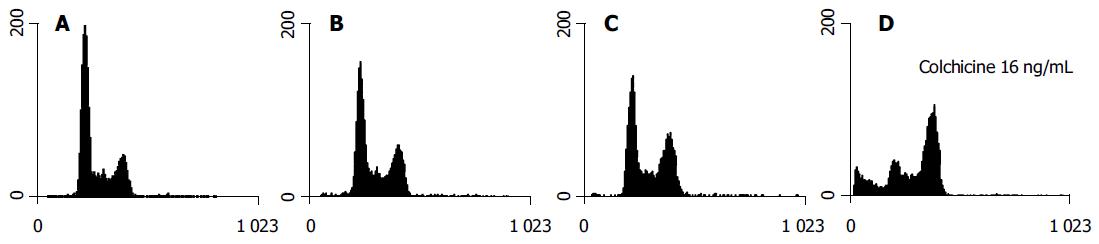
Although colchicine is reported to arrest mitosis, no HA22T/ VGH HCC cells were clearly seen in the G2/M phase 1-h treatment (data not shown). After 24 h of colchicine treatment, significant G2/M arrest was induced by higher doses (8 and 16 ng/mL) but not by doses, which were less than 8 ng/mL (Table 2). The DNA histograms after 24 h of treatment with varying doses of colchicine are shown in Figure 2.
The present study demonstrates that pretreatment with colchicine is capable of reducing the survival of irradiated human HCC HA22T/VGH cells. The combined effect varies with the dose of colchicine. Pretreatment with colchicine at lower doses (1-2 ng/mL) 1 h prior to RT yielded a synergistic effect on radiation-induced cytotoxicity. Higher doses gave only a mild additive effect. However, the G2/M arrest phenomenon was not found with low doses or short-term (1 h) colchicine treatment. Evident G2/M arrest was observed after treatment with higher doses (8 and 16 ng/mL) of colchicine for 24 h. Alteration of cell cycle distribution therefore may not play a major role in the radiosensitizing effect of colchicine in this combined therapy.
RT plays an important role in combined treatment for several malignant tumors[18,19]. However, the risk of radiation-induced toxicity limits its use in liver tumors[20]. While 3DRT is increasingly used for HCC, limiting the volume of irradiated normal liver and allowing adjustment of the radiation dose to improve the therapeutic index, it still has many limitations. Advanced liver cirrhosis or poor liver reserve is a common problem in patients with HCC[21]. The risk of hepatic failure after cytotoxic doses of RT therefore remains as a great problem. Radiosensitizers may give patients with poor liver reserve a better chance of controlling their tumor. Pentoxifylline and ganglioside GD3 have been shown to act as radiosensitizers in the human HCC cell line HepG2. Pentoxifylline may do so by causing cell cycle arrest in the G2/M phase. Ganglioside GD3 may have a dual action in producing radiosensitization by interacting with mitochondria and by inactivating NF-κB[22,23].
The safety and therapeutic range of colchicine are well established. The doses of colchicine (0-8 ng/mL) we used before RT are much lower than those in previous reports for other in vitro disease models[7]. Our results demonstrate that colchicine enhances the radiation effect in vitro, thus reducing the radiation dose needed to inhibit tumor growth. Most RT-related complications develop not early in the course of RT but rather after a high cumulative dose. Using colchicine at low doses yielded an SER of 1.21 at 1 ng/mL and 1.53 at 2 ng/mL. This means that if the original planned RT dose was 50 Gy, the same cytotoxic effect would be achieved by only 32.68 Gy after pretreatment with 2 ng/mL of colchicine. This reduced RT dose is close to 30 Gy, the threshold for whole liver irradiation, which may result in lethal liver damage with a 5% probability in 5 years (TD 5/5)[20].
Our results suggest that lower doses of colchicine sensitize HA22T/VGH HCC cells to radiation. At lower doses (1 and 2 ng/mL), 24 h of treatment with colchicine did not change the G2/M phase distribution significantly. At higher doses (8 and 16 ng/mL), the drug arrested that cell cycle at the G2/M phase. There was an accompanying evident sub-G1 peak noted on the DNA histogram (Figure 2). This implies that the synergistic effect of colchicine and RT may involve multiple mechanisms, perhaps including cell cycle regulation, induction of apoptosis, and others.
In conclusion, colchicine at optimal doses sensitizes human HA22T/VGH HCC cells to radiation by a mechanism other than cell cycle arrest at the G2/M phase.
The authors wish to thank Dr. Mary Jeanne Buttrey for critical reading and correction of the manuscript.
| 1. | Ben-Chetrit E, Levy M. Colchicine: 1998 update. Semin Arthritis Rheum. 1998;28:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 228] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Emmerson BT. The management of gout. N Engl J Med. 1996;334:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 312] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Yu TF, Gutman AB. Efficacy of colchicine prophylaxis in gout. Prevention of recurrent gouty arthritis over a mean period of five years in 208 gouty subjects. Ann Intern Med. 1961;55:179-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet's disease. Lancet. 1989;1:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 328] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Floreani A, Lobello S, Brunetto M, Aneloni V, Chiaramonte M. Colchicine in chronic hepatitis B: a pilot study. Aliment Pharmacol Ther. 1998;12:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Lee YM, Kaplan MM. Efficacy of colchicine in patients with primary biliary cirrhosis poorly responsive to ursodiol and methotrexate. Am J Gastroenterol. 2003;98:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Wallace SL, Singer JZ. Review: systemic toxicity associated with the intravenous administration of colchicine--guidelines for use. J Rheumatol. 1988;15:495-499. [PubMed] |
| 8. | El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Faivre J, Forman D, Esteve J, Obradovic M, Sant M. Sur-vival of patients with primary liver cancer, pancreatic can-cer and biliary tract cancer in Europe. Eur J Cancer. 1998;34:2184-2190. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 10. | El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 10.8] [Reference Citation Analysis (3)] |
| 11. | Nguyen MH, Keeffe EB. Treatment of Hepatocellular cancer. Gastrointestinal Cancers. London: Saunders 2003; 615. |
| 12. | Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, Sung JL. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Sinclair WK, Morton RA. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966;29:450-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 359] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Lin YM, Hu CP, Chou CK, O-Lee TW, Wuu KT, Chen TY, Peng FK, Liu TJ, Ko JL, Chang CM. A new human hepatoma cell line: establishment and characterization. Zhonghua MinGuo WeiSheng WuJi MianYiXue ZaZhi. 1982;15:193-201. [PubMed] |
| 16. | Chang C, Lin Y, O-Lee TW, Chou CK, Lee TS, Liu TJ, P'eng FK, Chen TY, Hu CP. Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol Cell Biol. 1983;3:1133-1137. [PubMed] |
| 17. | Hall EJ. Cell survival curves. 5 th ed. Philadelphia: Lippincott Williams & Wilkins 2000; 32-37. |
| 18. | Kuban DA, Dong L. High-dose intensity modulated radiation therapy for prostate cancer. Curr Urol Rep. 2004;5:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Olmi P, Fallai C, Cerrotta AM, Lozza L, Badii D. Breast cancer in the elderly: the role of adjuvant radiation therapy. Crit Rev Oncol Hematol. 2003;48:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3227] [Cited by in RCA: 3115] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 21. | Alsowmely AM, Hodgson HJ. Non-surgical treatment of hepatocellular carcinoma. Aliment Pharmacol Ther. 2002;16:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Wu DH, Liu L, Chen LH. Radiosensitization by pentoxifylline in human hepatoma cell line HepG2 and its mechanism. DiYi JunYi DaXue XueBao. 2004;24:382-385. [PubMed] |
| 23. | Paris R, Morales A, Coll O, Sánchez-Reyes A, García-Ruiz C, Fernández-Checa JC. Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J Biol Chem. 2002;277:49870-49876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Science Editor Guo SY Language Editor Elsevier HK