Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4154
Revised: January 10, 2005
Accepted: January 13, 2005
Published online: July 21, 2005
AIM: To investigate the effects of leptin (1-20 μg/kg) on acidified ethanol (AE)- and indomethacin (Indo)-induced gastric lesions in rats and compare it with ranitidine, lanso-prazole, and omeprazole and to determine its mechanisms of actions.
METHODS: Gastric ulcers, which were approximately 1 mm in width, formed in the glandular portion of the gastric mucosa produced by oral administration of either AE or Indo were taken as ulcer index. The inhibitory effect of subcutaneous administration of leptin, two proton pump inhibitors (PPIs) lansoprazole and omeprazole, or H2-receptor antagonist ranitidine 30 min before AE or Indo was evaluated. A radioimmunoassay was used to determine the PGE2 concentration in the homogenate of the glandular portion of the stomach. We performed histological study of the glandular stomach for the evaluation of total, acidic, and sulfated mucus content.
RESULTS: Subcutaneous administration of leptin, two PPIs lansoprazole and omeprazole or H2-receptor antagonist ranitidine 30 min before AE or Indo produced a dose-dependent and reproducible inhibition of gastric ulcers (GUs). This inhibition was found to be more potent than other antagonists used. In NG-nitro L-arginine methyl ester (L-NAME)-pretreated animals, the ulcer prevention ability of leptin in AE-induced ulcer was significantly reduced, compared to rats without L-NAME pretreatment. However, the ulcer prevention ability of leptin was not altered by L-NAME treatment in Indo-induced ulcers. Leptin produced a dose-dependent increase in PGE2 level in the gastric glandular tissues. Leptin also increased mucus secretion.
CONCLUSION: The results of the present study show that leptin inhibits GU formation by AE or Indo in a dose-dependent and reproducible manner in rats. The results also suggest that leptin prevents ulcer formation by increasing the activities of the cyclo-oxygenase and/or nitric oxide pathways and by increasing mucus secretion.
- Citation: Adeyemi EO, Bastaki SA, Chandranath IS, Hasan MY, Fahim M, Adem A. Mechanisms of action of leptin in preventing gastric ulcer. World J Gastroenterol 2005; 11(27): 4154-4160
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4154
Peptic ulcer is caused by a break in the mucosal integrity, close to the acid-secreting areas of the gastrointestinal tract. It is a disease that was characterized by remissions and exacerbations before the era of gastric acid secretion modifying drugs or Helicobacter pylori (H pylori). It is often located in the stomach (gastric ulcer, GU), in the proximal duodenum (duodenal ulcer), rarely in the esophagus, jejunum (distal to a gastrojejunal anastomosis) or the Meckel’s diverticulum, containing ectopic gastric mucosa. Development of a peptic ulcer depends on the balance between the known aggressive factors and mucosal defense mechanisms. Some of the aggressive factors are gastric acid[1,2], bile salts, abnormal motility[3], pepsin, use of nonsteroidal anti-inflammatory drugs (NSAID)[4], infection with micro-organisms (H pylori, herpes simplex and others). Mucus secretion, gastroduodenal bicarbonate production[5], prostaglandin synthesis[6], cholecys-tokinin and somatostatin[7], cellular regeneration, and normal tissue microcirculation protect against ulcer formation.
Leptin, a 167-amino acid peptide hormone, is secreted by the adipocytes into the circulation, lowers the body weight by decreasing appetite and increasing energy expenditure[8-10]. It has also been found to be present in the gastric mucosa[11], and this has been thought to control food intake in man. Another way of interpreting the presence of leptin in the gastric mucosa is to invoke its ability to function as a mucosal defense factor in the stomach. Indeed, a Polish group of workers showed recently that leptin has a gastro-protective effect in rats[12]. Much more recently, a Turkish group of workers showed that leptin has a gastro-protective effect on mucosal injury induced by ischemia reperfusion[13]. To date, there is a dearth of information in the literature about the mechanism by which leptin prevents ulcer formation in the stomach. Thus, the primary aim of this project was to investigate the effect of leptin on PGE2 synthesis on ulcer formation by indomethacin (Indo), a NSAID, or acidified ethanol (AE) in rats. The second aim was to study the role of leptin in relation to other established anti-ulcer agents, such as ranitidine[14] and PPIs[15] on peptic ulcer prevention in rats.
The following drugs and chemicals were used for the experiments: absolute alcohol (BDH, Poole, UK), hydrochloric acid (BDH), lansoprazole (Sigma, St. Louis, MO, USA), omeprazole (Astra, Uppsala, Sweden), Indo (Sigma, UK), L-NAME (Sigma, UK) and high sensitivity prostaglandin E2 Chemiluminescence Enzyme Immunoassay Kit (Assay Designs Inc., MI, USA). Leptin was a generous gift from Amgen Inc., USA. All solutions were freshly prepared and diluted in normal saline.
Wistar rats weighing 200-250 g were fasted for 18 h in wire mesh cages to avoid coprophagy. The animals were deprived of food but had free access to water ad libitum. The temperature of the animal room was maintained at 22 ± 2°C and a 12-12 h dark-light cycle was maintained. All animals used for the study had an ethical clearance from the Animal Users’ Committee of the Faculty of Medicine, United Arab Emirates University.
Indo (30 mg/kg) was administered orally to induce GUs in 18-h fasted Wistar rats. Gastric lesions were formed 6 h after Indo administration. The rats were divided into different groups of six animals each and Indo was given to each animal 30 min after administering leptin (1-50 μg/kg, subcutaneously) or normal saline (1 mL/kg, subcutaneously), and the animals were killed 6 h later by cervical dislocation and exsanguinations. The abdomen was incised, the stomach removed and cut open along the greater curvature and rinsed with water to remove any adherent food particles and mucus. The opened stomach was spread on a sheet of cork so as to have a clear macroscopic view of the gastric mucosa. The total lengths of the hemorrhagic lesions, which were approximately 1 mm in width formed in the glandular portion of the gastric mucosa, were taken as ulcer index. An observer unaware of the drug treatments confirmed the ulcer index. The percentage reduction of the ulcer index in the drug-treated groups was calculated from the saline-treated groups.
The ability of different doses of leptin (1-50 μg/kg) and a fixed dose of ranitidine (50 mg/kg), all administered subcuta-neously, to prevent the formation of Indo-induced GU was studied. The rats were killed 6 h after administering Indo by cervical dislocation and exsanguinations. The abdomen was incised and the stomach removed and the ulcer index was determined as described above. The reduction of the ulcer in the drug-treated groups was calculated as a percentage of the ulcer index in the saline-treated group.
In another experiment, 10 mg/kg each of lansoprazole and omeprazole, or 10 μg/kg of leptin was administered 30 min before the animals received Indo orally. The rest of the experiment and determination of the ulcer index were performed as described earlier.
AE was administered orally to induce GUs in rats. We used 60% ethanol in 150 mmol/L HCl as an ulcerogenic agent, based on earlier observation that ethanol 50% and above caused a reproducible model of gastric damage[16,17]. Gastric lesions were formed 1 h after administering AE. The rats were divided into different groups of six animals each and each group received either saline (1 mL/kg) or leptin (1-20 μg/kg), subcutaneously. Thirty minutes later, AE was given orally to each animal at a dose of 1 mL per rat and the animals were killed 1 h later by cervical dislocation and exsanguinations. The ulcer index was determined as described above.
In order to study the influence of prostaglandin on the cytoprotective activity of leptin, Indo was administered at a dose of 10 mg/kg subcutaneously (a dose, which inhibits prostaglandin synthesis, but does not induce gastric ulceration) to one group of animals followed by subcutaneous administration of leptin (10 μg/kg), 30 min later. AE was given to each animal 30 min after drug administration and the animals were killed 1 h later. The stomachs were subseq-uently removed to measure the ulcer index as described earlier.
In another group of animals, NG-nitro L-arginine methyl ester (L-NAME, 25 mg/kg), an inhibitor of nitric oxide synthase activity, was administered subcutaneously 15 min before giving leptin (10 μg/kg, subcutaneously). AE was given orally 30 min later and the animals were killed 1 h after ethanol administration to determine the ulcer index. In another group of L-NAME-pretreated animals, Indo was given orally instead of AE to induce ulcers, and the animals were killed 6 h later to determine the ulcer index.
The glandular portion of the control and leptin-treated rat stomachs were homogenized in 1.5 mL of Tris buffer (50 mmol/L; pH 7.5) containing 10 μg/mL Indo to prevent further cyclo-oxygenase activity at 0°C. The tissue homogenate was acidified by addition of 2 mmol/L HCl to a pH of 3.5, and allowed to settle at 4°C for 15 min before centrifuging the samples in a microcentrifuge at 1 000 r/min for 5 min to remove the tissue debris. The supernatant was harvested and stored at -20°C until assayed, usually within 24 h. A radioimmunoassay was used to determine the PGE2 concentration in the supernatant according to the instructions of the manufacturer (Assay Designs Inc., MI, USA). The assay is based on the competitive binding technique by which PGE2 present in a sample competes with a fixed amount of alkaline phosphatase-labeled PGE2 for sites on a mouse mAb. During incubation, the mouse mAb is bound to the goat anti-mouse antibody coated onto the microtiter well plate. Following a wash to remove excess conjugate and unbound sample, a substrate solution is added to the wells to determine the bound enzyme activity. Immediately following the color development, the luminescence is read in a luminometer. The intensity of the color is inversely proportional to the concentration of PGE2 in the sample. A standard curve was constructed from a series of known concentrations of PGE2 solution provided by the kit manufacturer, and the PGE2 concentrations of the unknown samples were determined by interpolation.
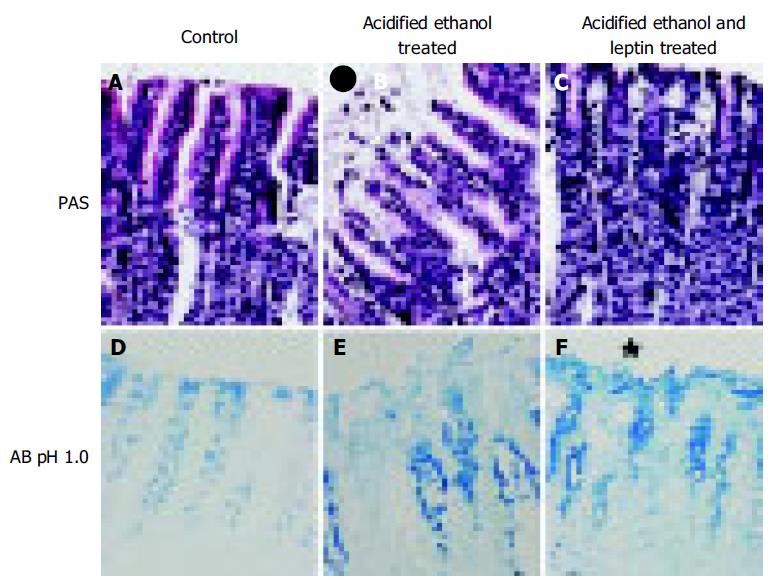
We performed histological study of the glandular stomach for the evaluation of total, acidic, and sulfated mucus content. Cross-sectional areas of the portion of the gland stained red by periodic acid Schiff (PAS) were measured to give the total glycoprotein present in the gastric pits. The Alcian Blue (AB) method at pH 1 was used to measure the sulfated macromolecular content in the gastric pits, whereas at pH 2.5 were used to measure the total acidic mucus content.
The glandular portion of the stomach was fixed in Zamboni fixative overnight. Smaller pieces cut from the glandular portion was dehydrated with ethanol and embedded in paraffin. Two series of sections (6 μmol/L thick) were made by cutting the block in a plane perpendicular to the mucosal surface. Sections, after being deparaffinized and hydrated to distilled water and placed in acetic acid solution, were stained with PAS and/or AB (pH 2.5 and 1). They were serially dehydrated in 95% ethyl alcohol, absolute alcohol, and xylene and mounted in resinous medium. The sections were evaluated in a randomized blinded fashion by a histologist who was unaware of the experimental protocol.
All results were expressed as mean±SE. Ulcer index obtained for test drugs and saline controls were compared using one-way ANOVA. A two-tailed P < 0.05 was considered significant.
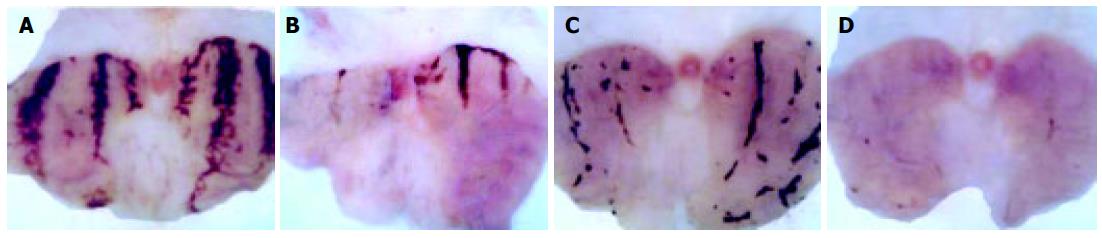
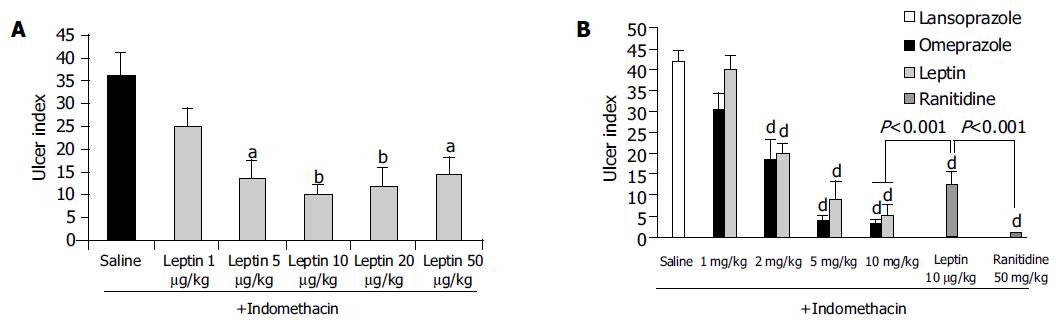
Indo caused a marked ulceration of the stomach in those rats given normal saline (Figures 1A-D). Figure shows hemorrhagic lesions on the glandular area of the rat stomach caused by AE (A) and Indo (C) in the absence (A and C) and presence (B and D) of 10 μg/kg leptin. A significant inhibition of the hemorrhagic lesions due to the leptin treatment was evident. When the animals were treated with leptin (1-50 μg/kg), there was a dose-dependent increase of the ulcer prevention ability of leptin, against Indo and formed a plateau at a dose of 20 μg/kg leptin. The ulcer index at each of the leptin concentrations from 5-50 μg/kg was significantly less (P < 0.05) than the ulcer index in normal saline-treated rats (Figure 2A).
Figure 2B reveals the degree of preventing Indo-induced ulcerations when the animals were treated with lansoprazole and omeprazole, each used at a dose of 1, 2, 5, and 10 mg/kg. There was a profound inhibition of ulcer formation at 10 mg/kg of both lansoprazole and omeprazole. The difference between the drug-treated group (lansoprazole or omeprazole at 2-10 mg/kg) and the saline-treated group was significant (P < 0.001); with lansoprazole showing a slightly more powerful inhibition than omeprazole but the difference between them was not statistically significant. The leptin ulcer inhibition at 10 μg/kg dosage was significantly less (P < 0.001) than that achieved with lansoprazole or omeprazole, each administered at 10 mg/kg (1 000 times the dose of leptin used; Figure 2B). When the rats were treated with 50 mg/kg of ranitidine (5 000 times the dose of leptin), the ulcer prevention activity was significantly higher (P < 0.001) than that achieved with 10 μg/kg leptin (Figure 2B).
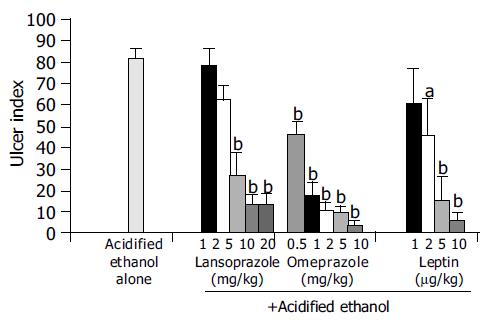
When the animals were treated with increasing doses of leptin (1-10 μg/kg), lansoprazole (1-20 mg/kg) and omeprazole (0.5-10 mg/kg) 30 min before they received AE, there was a dose-dependent inhibition of ulcer formation by leptin and each of the PPIs (Figure 3).
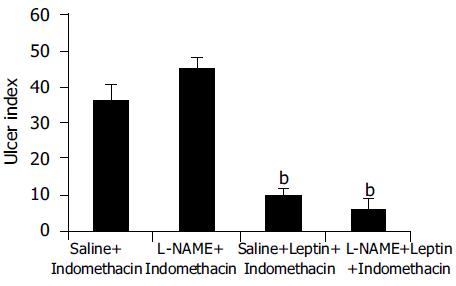
Leptin (10 μg/kg) produced a significant (P < 0.001) inhibition of Indo-induced ulcers in L-NAME-pretreated animals, which was not statistically significantly different from the ulcer index for leptin in animals without L-NAME pretreatment (Figure 4).
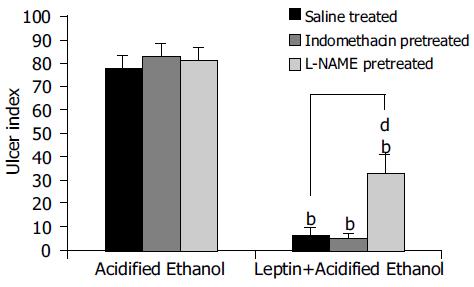
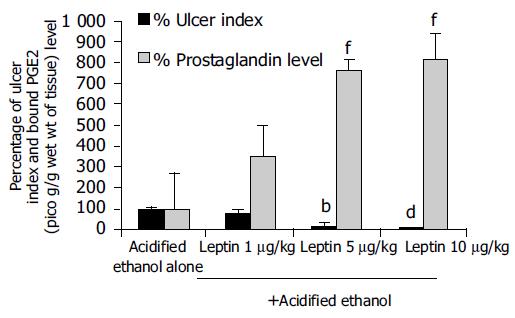
Leptin caused a profound ulcer inhibition, which was significantly greater (P < 0.001) for 10 μg/kg of leptin than saline-treated controls when AE was used to induce gastric ulceration in Indo-pretreated animals (Figure 5). When ulcer induction was performed with AE in L-NAME-pretreated rats, there was a significantly greater degree (P < 0.001) of GU inhibition by leptin than saline-treated controls (Figure 5). However, the inhibition by leptin of AE-induced ulcer formation was significantly less (P < 0.001) in the presence of L-NAME than in its absence (Figure 5). Injection of increasing doses of leptin to the animals before AE led to increasing degrees of peptic ulcer prevention and higher tissue concentrations of PGE2 (Figure 6). Leptin increased mucus secretion (Figures 7D, F) and prevented injury to the epithelial layer (Figures 7A-C) thus showing the involvement of mucus as the mechanism of gastroprotection.
Leptin is a peptide that is produced by the adipocytes and it regulates the function of the satiety center in the hypoth-alamus[8]. Although it has also been found in the stomach[11] its function in the stomach is not clear. In this study, we investigated the property of leptin as an ulcer-preventing agent in an experimental animal model. Our results showed that exogenous leptin demonstrated profound ulcer prevention ability in rats when their gastric mucosa was exposed to ulcer-inducing agents, such as Indo and AE. Exogenous administration of leptin increased the leptin levels in the blood plasma, fundus, and antrum of the stomach[18,19]. The basal leptin concentration did not prevent by itself the ulcerogenic effect of Indo or AE. However, exogenously administered leptin prevented this effect indicating a pharmacological action of leptin.
Indo is a well-known ulcerogenic agent. In this study, Indo produced hemorrhagic lesions in the gastric mucosa 6 h after its ingestion, which is in accord with previous reports[20-23]. In Indo-induced ulceration, the ulcer prevention ability of leptin was dose-dependent, reaching a maximum at 10 μg/kg. No further increase was observed when the leptin dose was doubled. Indeed, its ulcer prevention ability was reduced at a higher leptin dose of 50 μg/kg. This phenomenon may be explained by an increase in gastric acid secretion that was observed in this study (unpublished data). At this dosage, the leptin ulcer prevention ability was significantly less (P < 0.05) than that achieved with ranitidine, which was used at 50 mg/kg, 5 000 times the dose of leptin. However, the ulcer prevention ability of leptin at 10 μg/kg compares well with that of omeprazole or lansoprazole, each used at 10 mg/kg, 1 000 times the dose at which leptin was employed.
AE, a well-known ulcerogenic agent, was used in the study to produce gastric mucosal damage by causing areas of focal hyperemia and hemorrhage[22,24,25]. Moreover, intragastric ethanol increases vascular permeability and vascular damage in capillaries near the luminal surface and not in the deeper muscularis mucosa that might indicate a role for impaired blood flow in the production of AE-induced gastric lesions. Leptin demonstrated a profound ulcer prevention ability, which compared well with that of each of the PPIs, lansoprazole and omeprazole, when AE was employed as an ulcerogenic agent. This is in line with previous reports[12] that leptin has gastro-protective effect on the gastric mucosal injury induced by topical application of 75% ethanol.
It was interesting to note that the leptin ulcer prevention ability was slightly greater in AE- than in Indo-induced ulceration, although it did not reach statistical level of significance. This may be due to the fact that the AE-induced ulcers are formed superficially at the epithelial layer unlike that induced by Indo, which affects the deeper layer, the submucosa. This profound effect of inhibiting AE-induced ulcer formation by leptin was maintained, even when the animals were pretreated with low dose of Indo. However, a competition in the production of gastric mucosal PGE2 between Indo (inhibition) and leptin (stimulation) cannot be ruled out. In AE-induced ulceration, evaluation of the effect of leptin on gastric tissue prostaglandin synthesis revealed a dose-dependent increase of gastric mucosal PGE2 concentration[26]. This finding suggests that leptin might act in part via the cyclo-oxygenase pathway in preventing ulcer formation. Furthermore our findings that leptin stimulated mucus secretion support the notion that this peptide acted by stimulating PGE2 production. It has been reported previously that PGE2 stimulates mucus secretion[26-28]. In contrast to our results Brzozowski et al[12] reported that prostaglandins are not involved in the gastro-protective effect of leptin. The contradictory finding observed in our present results could be due to methodological differences in inducing ulcer.
The influence of leptin-induced histamine release, which has a protective effect on the gastric mucosa, was reported by several workers[13,29]. Morimoto et al[29] have reported that histamine release in the hypothalamus was significantly increased by leptin administration. Erkasap et al[13] have reported that leptin exerts a cytoprotective effect by increasing gastric tissue histamine content which in turn maintain the gastric mucosal blood flow, thereby suggesting that histamine is involved in the prevention of ischemia reperfusion-induced gastric mucosal injury. Goiot et al[30] reported that the co-existence of leptin and STAT-3 proteins in antral cells provide evidence for the involvement of leptin in the control of gastric secretions. Konturek et al[31] have investigated the effect of leptin on gastric acid secretion using H pylori-positive and -negative patients and reported that gastric meal-and CCK-induced leptin is capable of inhibiting basal and meal stimulated gastric H+ secretion in H pylori-positive patients. But in H pylori-negative patients the release of leptin was reduced in response to CCK and meal. We have shown that subcutaneous administration of low doses of leptin (1-50 μg/kg) potentiates the gastric acid stimulating effect of dimaprit, an H2 receptor agonist, and pentagastrin, a gastrin receptor agonist, in anaesthetized rats (results not shown). The variation in our results and those of others may be due to the methodological approach towards the evaluation of leptin effect. Taken together our data and those of Azuma et al[32] Morimoto et al[29] Erkasap et al[13] and Konturek et al[19] suggested that, in addition to the local effect of leptin, a generalized effect of leptin could not be ruled out.
Pretreatment of the animals with L-NAME (25 mg/kg, subcutaneously), an inhibitor of the nitric oxide synthase, before AE-induced gastric ulcerations, reduced the peptic ulcer prevention ability of leptin (10 μg/kg) significantly. However, it did not blot it out completely. This finding suggests that the peptic ulcer prevention ability of leptin does not depend solely on the nitric oxide pathway. This again is in line with the observation of Brzozowski et al[12] that L-NAME reduced the ulcer-preventing ability of leptin against AE. Brzozowski et al[33] have also investigated the effect of centrally and intraperitoneally administered leptin and reported that its gastro-protective action, accompanied by increased gastric blood flow and increased plasma gastrin levels, depends on vagal activity and involves hyperemia mediated by NO.
Our results may have consequences for the clinical practice. Based on our data, one would want to recommend the use of leptin subcutaneously in a pilot study in humans, in order to assess its efficacy as an ulcer prevention drug in patients in the intensive care unit, who are prone to develop stress ulcers[34] during the course of their treatment. Another potential area for its use is among patients with active rheumatoid arthritis[35] who need to use NSAID for their inflammatory joint disease. This is mainly because the existing epidemiological data support a causal relationship between NSAID use and the development of clinically important ulcer disease or its complications, including upper GI bleeding.
In conclusion, we have shown that leptin, at low doses (1-10 μg/kg), has ulcer prevention ability, which is comparable to that of ranitidine, an H2-receptor antagonist or omeprazole and lansoprazole. The ulcer prevention ability of leptin varies according to the ulcerogenic agent used. It is more effective in inhibiting AE-induced than Indo-induced ulcers. The ulcer prevention ability of leptin in AE-induced ulcer involves the cyclooxygenase and the nitric oxide pathways, whereas it acts only via the cyclooxygenase pathway in preventing Indo-induced ulcers.
We would like to thank Amgen Inc., USA, for kindly donating the leptin, which was used in this study. We would also like to thank Dr. Eric Mensah-Brown for his help in studying the photomicrographs and Mr. Naseer Omar for assisting in animal experiments and Dr. Edward Adyemi who passed away at the end of the year 2004.
| 1. | Magalhaes AF, Macedo C, Hauck JR, Carvalhaes A, De Nucci G, Magna LA, Pedrazzoli J. Acid suppression with ranitidine plus oral triple therapy improves ulcer healing but not Helicobacter pylori eradication. Hepatogastroenterology. 1998;45:2161-2164. [PubMed] |
| 2. | Brown LF, Wilson DE. Gastroduodenal ulcers: causes, diagnosis, prevention and treatment. Compr Ther. 1999;25:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Azuma T, Dojyo M, Ito S, Yamazaki Y, Miyaji H, Ito Y, Suto H, Kuriyama M, Kato T, Kohli Y. Bile reflux due to disturbed gastric movement is a cause of spontaneous gastric ulcer in W/Wv mice. Dig Dis Sci. 1999;44:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Gaw AJ, Williams LV, Spraggs CF, Jordan CC. Role of pepsin in the development of indomethacin-induced antral ulceration in the rat. Aliment Pharmacol Ther. 1995;9:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Hogan DL, Ainsworth MA, Isenberg JI. Review article: gastroduodenal bicarbonate secretion. Aliment Pharmacol Ther. 1994;8:475-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Peskar BM, Maricic N. Role of prostaglandins in gastroprotection. Dig Dis Sci. 1998;43:23S-29S. [PubMed] |
| 7. | Brzozowski T, Konturek PC, Konturek SJ, Kwiecién S, Pajdo R, Brzozowska I, Hahn EG. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998;27 Suppl 1:S125-S137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8941] [Article Influence: 279.4] [Reference Citation Analysis (0)] |
| 9. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3184] [Cited by in RCA: 3088] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 10. | Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2882] [Cited by in RCA: 2838] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 11. | Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y. The stomach is a source of leptin. Nature. 1998;394:790-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 772] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Duda A, Pierzchalski P, Bielański W, Hahn EG. Leptin in gastroprotection induced by cholecystokinin or by a meal. Role of vagal and sensory nerves and nitric oxide. Eur J Pharmacol. 1999;374:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Erkasap N, Uzuner K, Serteser M, Köken T, Aydin Y. Gastroprotective effect of leptin on gastric mucosal injury induced by ischemia-reperfusion is related to gastric histamine content in rats. Peptides. 2003;24:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Lazzaroni M, Bianchi Porro G. Prophylaxis and treatment of non-steroidal anti-inflammatory drug-induced upper gastrointestinal side-effects. Dig Liver Dis. 2001;33 Suppl 2:S44-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Warzecha Z, Dembiński A, Brzozowski T, Ceranowicz P, Dembiński M, Stachura J, Konturek SJ. Histamine in stress ulcer prophylaxis in rats. J Physiol Pharmacol. 2001;52:407-421. [PubMed] |
| 16. | Nishida A, Takinami Y, Yuki H, Kobayashi A, Akuzawa S, Kamato T, Ito H, Yamano M, Nagakura Y, Miyata K. YM022 (R)-1-[2,3-dihydro-1-(2'-methylphenacyl)-2-oxo-5-phenyl- 1H-1,4-benzodiazepin-3-yl]-3-(3-methylphenyl)urea], a potent and selective gastrin/cholecystokinin-B receptor antagonist, prevents gastric and duodenal lesions in rats. J Pharmacol Exp Ther. 1994;270:1256-1261. [PubMed] |
| 17. | Mercer DW, Cross JM, Barreto JC, Strobel NH, Russell DH, Miller TA. Cholecystokinin is a potent protective agent against alcohol-induced gastric injury in the rat. Role of endogenous prostaglandins. Dig Dis Sci. 1995;40:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Konturek PC, Brzozowski T, Sulekova Z, Meixner H, Hahn EG, Konturek SJ. Enhanced expression of leptin following acute gastric injury in rat. J Physiol Pharmacol. 1999;50:587-595. [PubMed] |
| 20. | Kasuya Y, Urushidani T, Okabe S. Effects of various drugs and vagotomy on indomethacin-induced gastric ulcers in the rat. Jpn J Pharmacol. 1979;29:670-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ueki S, Takeuchi K, Okabe S. Gastric motility is an important factor in the pathogenesis of indomethacin-induced gastric mucosal lesions in rats. Dig Dis Sci. 1988;33:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bastaki SM, Chandranath SI, Singh J. Comparison of the antisecretory and antiulcer activity of epidermal growth factor, urogastrone and transforming growth factor alpha and its derivative in rodents in vivo. Mol Cell Biochem. 2002;236:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Chandranath SI, Bastaki SM, Singh J. A comparative study on the activity of lansoprazole, omeprazole and PD-136450 on acidified ethanol- and indomethacin-induced gastric lesions in the rat. Clin Exp Pharmacol Physiol. 2002;29:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Lacy ER, Ito S. Microscopic analysis of ethanol damage to rat gastric mucosa after treatment with a prostaglandin. Gastroenterology. 1982;83:619-625. [PubMed] |
| 25. | Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 340] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Bastaki SMA, Chandranath SI, Adem A. The role of pros-taglandin E2 in thelcer preventing ability of leptin. Gut. 2004;53:A77. |
| 27. | Wallace JL, Tigley AW. Review article: new insights into prostaglandins and mucosal defence. Aliment Pharmacol Ther. 1995;9:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Bastaki SM, Wallace JL. Pathogenesis of nonsteroidal anti-inflammatory drug gastropathy: clues to preventative therapy. Can J Gastroenterol. 1999;13:123-127. [PubMed] |
| 29. | Morimoto T, Yamamoto Y, Yamatodani A. Leptin facilitates histamine release from the hypothalamus in rats. Brain Res. 2000;868:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Goïot H, Attoub S, Kermorgant S, Laigneau JP, Lardeux B, Lehy T, Lewin MJ, Bado A. Antral mucosa expresses functional leptin receptors coupled to STAT-3 signaling, which is involved in the control of gastric secretions in the rat. Gastroenterology. 2001;121:1417-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Konturek JW, Konturek SJ, Kwiecień N, Bielański W, Pawlik T, Rembiasz K, Domschke W. Leptin in the control of gastric secretion and gut hormones in humans infected with Helicobacter pylori. Scand J Gastroenterol. 2001;36:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Brzozowski T, Konturek PC, Pajdo R, Kwiecień S, Ptak A, Sliwowski Z, Drozdowicz D, Pawlik M, Konturek SJ, Hahn EG. Brain-gut axis in gastroprotection by leptin and cholecystokinin against ischemia-reperfusion induced gastric lesions. J Physiol Pharmacol. 2001;52:583-602. [PubMed] |
| 34. | Overmier JB, Murison R. Anxiety and helplessness in the face of stress predisposes, precipitates, and sustains gastric ulceration. Behav Brain Res. 2000;110:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Hochberg MC. Association of nonsteroidal anti-inflamma-tory drugs with upper gastrointestinal disease: epidemio-logical and economic considerations. J Rheumatol. 1992;19:63-67. |
Science Editor Guo SY Language Editor Elsevier HK