Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3619
Revised: July 18, 2004
Accepted: August 30, 2004
Published online: June 21, 2005
AIM: To investigate the inhibitory effect of Chinese herbal medicine on the transcription of hepatitis C virus (HCV) structural gene in Hela D cells.
METHODS: Hela cell line was transfected with recombinant pBK-CMV-HCV containing HCV structural gene by Lipofec-tamine. RT-nested-PCR and Western blot assay were used to testify the HCV gene expression in Hela cells. The Hela cells expressing HCV structural protein were named Hela D cells. Prescriptions of Xiao chaihu Decoction (XCHD), Fufang Huangqi (FFHQ) and Bingganling (BGL) were respectively added to Hela D cells in various concentrations. Semi-quantitative RT-nested-PCR product analysis was performed according to the fluorescent density between HCV DNA band and GAPDH DNA band in gel electrophoresis after screened.
RESULTS: Recombinant pBK-CMV-HCV could correctly express the HCV structural gene in Hela D cells. After co-culture of Hela D cells with three prescriptional different concentrations for 48 h respectively, the transcription of HCV gene decreased with increasing of the concentration of each prescription. The lightness ratio of HCV product bands to GAPDH product bands was 0.24, 0.10 and 0.12 in Hela D cells incubated with 0.1 g/mL of XCHD, FFHQ and BGL respectively and the lightness ratio HCV product bands to GAPDH product bands was 0.75, 0.67 and 0.61 respectively in the control cells.
CONCLUSION: The prescriptions of XCHD, FFHQ and BGL partly inhibit the transcription of HCV structural gene in Hela D cells.
-
Citation: Dou J, Chen Q, Wang J. Inhibition effect of Chinese herbal medicine on transcription of hepatitis C virus structural gene
in vitro . World J Gastroenterol 2005; 11(23): 3619-3622 - URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3619.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3619
Hepatitis C virus (HCV) is the major cause of non-A, non-B viral hepatitis and 60% of patients infected with HCV become chronic hepatitis C. Although the cloning of HCV genome was discovered more than a decade ago and researchers have made effects to find novel agents for prevention and treatment of hepatitis C[1,2], there is no efficient method to control or eradicate this disease yet, as investigation of the HCV life cycle has been limited by lack of an efficient cell culture system for HCV infection and replication[3]. Recently, it was reported that prescriptions of XCHD, FFHQ and BGL are potent in treating patients with hepatitis C[4-6]. However, the mechanism of these Chinese herbal medicines against HCV is not clear. Because of no economical and suitable animal model for HCV infection research except for chimpanzee, the cell model transfected with HCV genes becomes necessary for screening drugs for HCV[7]. Traditional Chinese herbal medicine is a great treasure-house. Using modern experiment methods and traditional Chinese medicine theory, it is possible to discover novel effect of Chinese herbal medicine on hepatitis C.
In this investigation, we established model of Hela D cells transfected with HCV structural genes and tried to select Chinese herbal medicine for inhibition of HCV transcription in Hela D cells in order to provide experimental data for clinical therapy of hepatitis C.
Hela cell line was incubated at 37 °C in 50 mL/L CO2 atmosphere in RPMI1640 (Gibco-BRL, USA) supplemented with 10% fetal bovine serum, 100 units/mL penicillin G sodium and 100 µg/mL streptomycin sulfate.
Recombinant plasmid pBK-CMV-HCV containing HCV structural genes (1-1686 base pair, gene type 1b), was kindly gifted by Dr. Chen Zhi in the Medical College of Zhejiang University.
XCHD composed of Chaihu (9g), Huangqi (6g), banxia (6g), Zheganchao (3g), shengjiang (6g) and Dazao (4 pieces), was purchased from Jiangsu Chinese Herbal Medicine Storyhouse. These raw drugs were mixed in water for 30 min and fried 1 h with small blaze on stove. The original fluid drug was filtered through four layer gauzes and then stored at 4 °C for use[8]. FFHQ was composed of Huangqi (45 g), Chaihu (12 g), Dansheng (20 g), Cheshao (15 g), Yujing (12 g) and Danpi (12 g). The original fluid drug was made as XCHD. BGL supplied by Professor Xue Boyu in Nanjing Traditional Chinese Medical University, was composed of Shuiliujiao, Hu Zhang, Cheshao, Huangqi, Jujizi, Chaihu, Chenpi, Fuling and Jiguchao.
All primers were synthesized by Shengon in Shanghai, China. The sequence of the first PCR forward primer for HCV structural gene was 5’ CGC GCG ACT AGG AAG ACT TTC 3’ and that of reverse primer was 5’ ATA TAC CCC ATG ACC TCG GC 3’. The sequence of the second PCR forward primer was 5’ AGG AAG ACT TCC GAG CGG GTC 3’ and that of reverse primer was 5’ GAG CCA TCC TGC CCA CCC CA 3’. The sequence of the forward primer for house-keeping gene GAPDH was 5’ TCC CAT CAC CAT CTT CCA 3’ and that of reverse primer was 5’ CAT CAC GCC ACA GTT TCC 3’.
Seventy percent confluent Hela cells in complete medium were transferred to a six well plate for 24 h. Mixture of the LipofectamineTM 2000 reagent and HCV1b genotype recombinant plasmid pBK-CMV-HCV was incubated for 20 min at room temperature and then mixture were directly put into Hela cells after the medium was removed from plates and then the plates were incubated at 37 °C in 50 mL/L CO2. Growth medium was replaced after incubation for 4 h, followed by selection with 600 µg/mL of G418 after 24 h[9]. Hela cells were transfected with blank plasmid as control. After seven days, G418-resistant clones were selected, clonally isolated and screened for Western blot assay, cytotoxicity assay and inhibition of transcription of HCV structural gene, respectively. The Hela cells expressing HCV structural protein were named Hela D cells.
Each kind of Chinese herbal medicine was put into Hela D cell culture medium for 72 h separately and the final concentration was 1.0, 0.8, 0.6, 0.4 and 0.2 g/mL, respectively. The cytotoxicity of Chinese herbal medicine to Hela D cells was observed under microscope and by MTT methods[10]. After Hela D cells were incubated for 10 h, 200 μL of vsarious concentrations of Chinese herbal medicine (0.1, 0.01, 0.001 g/mL) was respectively added to the culture medium in a six well plate for an additional 48 h incubation and 200 μL PsBS was added to Hela D cells as control.
Total RNA was isolated from Hela D cells with TRIzol reagent. Before extraction, 100 μL reaction buffer (10 mmol/L MgCl2, 0.1 mmol/L DTT, 1 u/mL RNasin, RNase-free DNaseI) was added to 2×106 Hela D cells and stored at 37 °C for 1 h to remove all contaminated DNA and then the extraction process was performed according to the TRIzol protocol[11].
cDNA was prepared by reverse transcription of 2 μg total RNA and then was amplified by nested PCR with two different primers to detect the transcriptions of HCV structural and GAPDH gene. In PCR amplified products, the GAPDH gene was used as an internal control of HCV gene[12]. PCR products were checked by gel electrophoresis and semiquantitative RNA analysis was performed according to the fluorescent density between HCV DNA band and internal control GAPDH gene band in gel electrophoresis.
Whole cell extract was prepared by protein extraction buffer (Novagen, Germany) according to the manufacturer’s protocol. Western blot was performed after 12% SDS-polyacrylamide gel electrophoresis using Western Breeze kit (Invitrogen, CA). Briefly, proteins (15 µg/lane) were electrotransferred onto nitrocellulose membrane. The membrane was blocked with blocking solution 30 min on a rotary shaker and washed twice with water and subsequently incubated with primary antibodies for 1 h. The membrane was washed thrice for 5 min with antibody washing solution. The rest of the steps were performed according to the kit’s protocol.
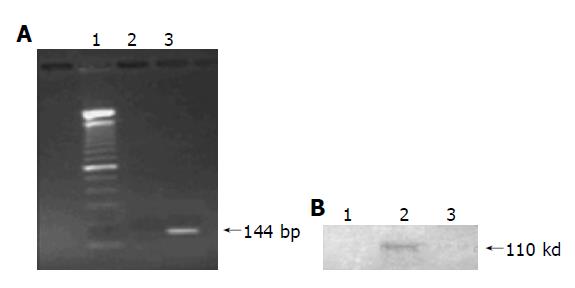
Total RNA was isolated from Hela D cells and Hela cells respectively with TRIzol reagent and cDNA was then amplified by nested PCR with two different primers. HCV structural gene was expressed in Hela D cells because there was a 144 bp band in the extracts from Hela D cells (Figure 1). However, there was no band in the extracts from Hela cells. Under similar conditions, there was no protein expression in Hela cells transfected with blank plasmid or Hela cells without any transfection.
When the concentration of Chinese herbal medicine gradually decreased, its cytotoxicity to Hela D cells was also decreased by degrees. When the concentration of Chinese herbal medicine was maintained at 0.2 g/mL in Hela D cells culture medium for 72 h, the survival rate of Hela D cells reached more than 99% (Table 1).
| Herbs | 1 (g/mL) | 0.8 (g/mL) | 0.6 (g/mL) | 0.4 (g/mL) | 0.2 (g/mL) |
| XCHD | 22.57 | 48.63 | 73.25 | 89.86 | 99.97 |
| FFHQ | 25.36 | 57.34 | 76.28 | 92.57 | 99.98 |
| BGL | 21.67 | 47.35 | 79.24 | 94.31 | 99.97 |
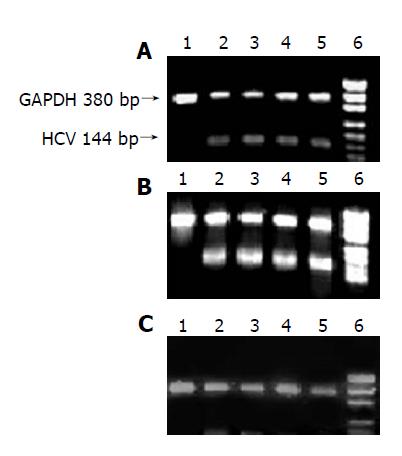
When the concentration of Chinese herbal medicine was increased, the transcription of HCV gene gradually decreased and its lightness of HCV band was weak, but the lightness of GAPDH band had no change, suggesting that XCHD, FFHQ and BGL could inhibit the transcription of HCV gene and had no effect on GAPDH in Hela D cells (Figure 2).
It is very difficult to establish a culture system in vitro for HCV replication because most cell lines are not sensitive to HCV infection and HCV in cell line cannot be cultured for a long time. The problem of successive incubation of normal liver cells is still not solved. Cell models transfected with HCV gene or part of gene become an effective method in research of HCV replication, gene transcription, protein expression and drug screening for treating hepatitis C. Some cell lines can be used as important tools to investigate structural and functional properties of HCV core protein and may be useful in evaluating gene therapeutic strategies against HCV[13,14]. Some researches have focused on Hela cell in recent years[15,16]. Ide et al[17], established a Hela cell model which can successfully express HCV NS 5A gene. It was reported that Hela cells transfected with non-structural gene and structural gene are used in research of the function of HCV proteins[7,11]. Based on these research reports, we used the Hela cell line as a cell model for selection of Chinese herbal medicine against HCV.
XCHD, FFHQ and BGL are effective prescriptions of Chinese herbal medicine in treating hepatitis C patients[4-6]. These prescriptions, however, lack laboratory evidence to support their clinical therapeutic effect on HCV. We employed molecular biological methods to establish Hela D cell model by transfection of HCV structural gene, which could stably express most HCV structural genes.
The cytotoxicity assay of XCHD, FFHQ and BGL to Hela D cells showed that the concentration of 0.2 g/mL Chinese herbal medicine had no cytotoxicity to Hela D cells after incubated for 72 h. The inhibitory effect of Chinese herbal medicine on HCV gene expression showed that the three prescriptions can partly inhibit the transcription of HCV structural gene because the transcription quantity of HCV gene is decreased as their concentration is increased. The efficient inhibitory effect was achieved at the highest dose of 0.1 g/mL of different Chinese herbal medicine.
The mechanism of XCHD, FFHQ and BGL underlying inhibition of HCV gene expression is still not clear. Based on the traditional Chinese medicine theory, we consider that Chinese herbal medicine can change the internal environment of cells, where recombinant pBK-CMV-HCV does not make use of synthetic enzymes and proteins for HCV structural gene mRNA transcription. There might be some pathways that inhibit HCV mRNA transcription in Hela D cells.
In conclusion, Hela D cell model is very useful for observation of HCV transcription, protein expression and for screening new drugs against HCV.
| 1. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 681] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 2. | Dou J, Liu K, Chen Z, Wo J, Liu Y, Xu C, Chen M, Jin J, He N. Experimental study of immunization of mice with hepatitis C virus genetic vaccine constructs. Zhonghua NeiKe ZaZhi. 1999;38:390-392. [PubMed] |
| 3. | Moradpour D, Gosert R, Egger D, Penin F, Blum HE, Bienz K. Membrane association of hepatitis C virus nonstructural proteins and identification of the membrane alteration that harbors the viral replication complex. Antiviral Res. 2003;60:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Li FC, Wei JB. Survey of Xiao Chai Hu Decoction in clinical application. Res Traditional Chinese Med. 1998;14:60-62. |
| 5. | Ma SY, Pei ZG. Treatment 46 cases of hepatitis C with method of integrated traditional and western medicine. Chin J Integrated Traditional Western Medi. 1996;16:558-560. |
| 6. | Cheng WP, Zhou ZG, Wu PL, Li B. Treatment 13 cases of hepatitis C with Bing Gan Ling. Information Traditional Chin Medi. 1997;14:23-25. |
| 7. | Ikeda M, Yi M, Li K, Lemon SM. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J Virol. 2002;76:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 330] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Dou J, Wu MY. Effect of si jun zi tang on the macrophage cytotoxic activity in mice. ZhongXiYi JieHe ZaZhi. 1990;10:612-613, 582. [PubMed] |
| 9. | Sun BS, Pan J, Clayton MM, Liu J, Yan X, Matskevich AA, Strayer DS, Gerber M, Feitelson MA. Hepatitis C virus replication in stably transfected HepG2 cells promotes hepatocellular growth and tumorigenesis. J Cell Physiol. 2004;201:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39998] [Article Influence: 930.2] [Reference Citation Analysis (1)] |
| 11. | Dou J, Liu K, Chen Z, Wo J, He N, Liu Y, Zhang M, Wang X, Xu C. Effect of immunization in mice with recombinant DNA encoding the hepatitis C virus structural protein. Chin Med J (Engl). 1999;112:1036-1039. [PubMed] |
| 12. | Kim JS, Ryu J, Hwang SB, Lee SY, Choi SY, Park J. Suppression of ceramide-induced cell death by hepatitis C virus core protein. J Biochem Mol Biol. 2004;37:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Moradpour D, Englert C, Wakita T, Wands JR. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology. 1996;222:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Yamaguchi R, Momosaki S, Gao G, Hsia CC, Kojiro M, Scudamore C, Tabor E. Truncated hepatitis C virus core protein encoded in hepatocellular carcinomas. Int J Mol Med. 2004;14:1097-1100. [PubMed] |
| 15. | Mizuno M, Yamada G, Tanaka T, Shimotohno K, Takatani M, Tsuji T. Virion-like structures in HeLa G cells transfected with the full-length sequence of the hepatitis C virus genome. Gastroenterology. 1995;109:1933-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Hassan M, Ghozlan H, Abdel-Kader O. Activation of RB/E2F signaling pathway is required for the modulation of hepatitis C virus core protein-induced cell growth in liver and non-liver cells. Cell Signal. 2004;16:1375-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Ide Y, Tanimoto A, Sasaguri Y, Padmanabhan R. Hepatitis C virus NS5A protein is phosphorylated in vitro by a stably bound protein kinase from HeLa cells and by cAMP-dependent protein kinase A-alpha catalytic subunit. Gene. 1997;201:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |