Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3570
Revised: July 13, 2004
Accepted: December 10, 2004
Published online: June 21, 2005
AIM: To investigate the oxidative-stress-related changes in rats with portal hypertension with particular emphasis on nitric oxide (NO) and trace metals.
METHODS: Cirrhosis was induced by partial portal vein ligation (PVL) in Wistar rats. The lipid peroxidation marker (malondialdehyde, MDA), antioxidant defense enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione (GSH), and agents known to have antioxidant features including nitric oxide (NO), zinc (Zn), copper (Cu) were determined both in serum and in liver tissue at 4 wk after surgery in PVL and sham-operated rats. Portal pressure of all experimental animals was measured. MDA was detected by thiobarbituric acid reactivity assay. SOD activity was determined by inhibition of nitroblue tetrazolium reduction with xanthine/xanthine oxidase used as a superoxide generator. CAT activity was determined by the breakdown of hydrogen peroxide. GSH concentrations were measured by using metaphosphoric acid for protein precipitation and 5’-5’-dithio-bis-2-nitrobenzoic acid for color development. NO was detected by the Griess method after reduction of nitrate to nitrite with nitrate reductase, and the concentrations of Zn and Cu were measured by a Shimadzu 680 AA atomic absorption spectrometer. Histopathological confirmation was done under light microscope. Statistical analyses were done by Student’s t-test, and significance of the difference was tested by the unpaired Mann-Whitney test. P<0.05 was considered statistically significant.
RESULTS: Histopathological studies confirmed PVL-induced cirrhotic changes. There was a statistically significant difference in portal pressure between PVL and control groups (P<0.001). The results showed significant increases in the levels of MDA and NO in both tissue and serum (P<0.05 and P<0.001, respectively in tissue; P<0.001 for each in serum), and Zn only in tissue (P<0.001) in rats with PVL compared with sham-operated rats. Besides, PVL rats exhibited reduced plasma and tissue GSH, CAT, SOD (P<0.001 for each). Serum and tissue Cu concentration did not change.
CONCLUSION: Our findings suggest that PVL in rats induces important biochemical and molecular changes related to oxidative stress in the liver.
- Citation: Izzet T, Osman K, Ethem U, Nihat Y, Ramazan K, Mustafa D, Hafize U, Riza KA, Birsen A, Habibe G, Seval A, Gonul S. Oxidative stress in portal hypertension-induced rats with particular emphasis on nitric oxide and trace metals. World J Gastroenterol 2005; 11(23): 3570-3573
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3570.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3570
A role of reactive oxygen species in the development of hyperdynamic circulation in portal hypertension has been proposed[1,2]. However, evaluation of the limited available data indicates that it is premature to conclude that oxidative stress has a primary role in cirrhotic hepatofibrosis and development of portal hypertension.
Portal hypertension is often accompanied with a hyperdynamic circulation state. Some reports have suggested that nitric oxide (NO), a vasodilatory agent, plays an important role in this hyperdynamic state[3]. On the other hand, NO is also known as an antioxidant.
In the pathogenesis of cirrhosis and development of portal hypertension, the role of trace elements has been described recently[4]. Among these, zinc (Zn) and copper (Cu) are essential trace elements and function as co-factors of antioxidant enzymes.
Oxidative stress in liver disease should be evaluated not only with lipid peroxidation parameters and antioxidant defense enzymes, but with agents known to have antioxidant features as well. The current literature is insufficient at this point as studies on the role of oxidative stress in cirrhosis and portal hypertension approached the subject from just one perspective.
In the present study, our purpose was to investigate the oxidative stress-related changes in portal hypertension-induced rats with particular emphasis on NO, Zn, and Cu, which are known as antioxidant agents.
The study was conducted with the approval of our institution’s ethics committee, and all experimental procedures were done according to the standards of Animal Care and Use Committee[5]. Thirty male Wistar Albino rats weighing 220-310 g were divided into control group (n = 10) and experimental group (n = 20). The animals were fed on standard laboratory diet and water ad libitum before and after surgery. All animals were anesthetized with ether to undergo a midline laparotomy. A sham laparotomy was performed in the control group. Portal hypertension was induced by partial stenosis of portal vein, and also the portal pressure of all experimental animals was measured according to a procedure described previously[6]. The animals were killed 4 wk later to observe the changes in the liver tissue.
Under ether anesthesia, 5 cm3 (3-7 cm3) of blood was taken by cardiac puncture following exploration of the thorax. Then, a laparotomy was done and the liver was excised and saved for biochemical analysis and histopathological confirmation.
Blood samples collected in heparinized vacutainer tubes were immediately transported to the laboratory in a cooler with ice. Upon arrival, plasma was separated by centrifugation (+4 °C, 3000 r/min, 10 min), and divided into 0.5-1.0 mL aliquots, placed in cryovials, and stored at -70 °C until analyzed. Erythrocytes were washed thrice in 5 mL saline, hemolyzed by diluting fourfold with water and glutathione (GSH) was studied in erythrocytes in the same day. Each plasma sample was divided into four aliquots. The first aliquot was saved until analysis of plasma NO, the second aliquot was used for Zn and Cu analyses, and the other two aliquots were used for estimation of plasma malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) levels in 1-wk period.
The liver tissues were weighed, washed in 0.9% NaCl, and homogenized in ice-cold 0.15 mol/L 100 KCl g/L. Twenty percent of homogenates were obtained and sonicated twice at 30-s intervals at 4 °C. Homogenates were centrifuged at >10000 g for 15 min at 4 °C. All biochemical parameters in homogenates were studied in the same day.
Lipid peroxidation MDA, as an end product of fatty acid peroxidation, was detected in plasma and liver homogenates by thiobarbituric acid reactivity assay as previously described[7]. The total protein concentration was measured by the method of Lowry et al[8].
Nitric oxide Plasma and tissue concentrations of NO were measured as its stable metabolites, nitrate and nitrite. Nitrate was first reduced by nitrate reductase to nitrite and then nitrite was determined spectrophotometrically by the Griess reaction[9]. Griess reagent, the mixture (1:1) of 0.2% N-(1-naphthyl)-ethylene-diamine and sulfanilamide in 5% phosphoric acid, gave red-violet diazo dye with nitrite, and was detected in the visible range at 540 nm.
Cu–Zn superoxide dismutase (Cu-Zn SOD) Plasma and tissue Cu-Zn SOD activities were determined by the method of Sun et al[10], by inhibition of nitroblue tetrazolium reduction with xanthine/xanthine oxidase used as a superoxide generator. One unit of SOD was defined as the amount of protein that inhibited the rate of NBT reduction by 50%.
Catalase CAT activity was determined by the breakdown of hydrogen peroxide catalyzed by CAT enzyme[11].
Glutathione Erythrocyte and tissue GSH concentrations were measured according to the method of Beutler et al[12], using metaphosphoric acid for protein precipitation and 5’-5’-dithio-bis-2-nitrobenzoic acid for color development.
Zn and Cu The concentrations of Zn and Cu were measured by a Shimadzu 680 AA atomic absorption spectrometer. The concentrations were expressed as nanogram per milliliter and microgram per deciliter, respectively.
Histopathological confirmation was done under light microscope, after the samples were sectioned and stained with hematoxylin and eosin.
All values were expressed as mean±SD. Statistical analyses were done by SPSS-programmed Student’s t-test at 11.5 version. The significance of the difference was tested by the unpaired Mann-Whitney test. P<0.05 was considered statistically significant.
There was a statistically significant difference in portal pressure between portal vein ligation (PVL) and control groups (18.4±1.7 cm saline vs 10.3±0.8 cm saline, P<0.001).
Histopathological studies confirmed PVL-induced cirrhotic changes. Macroscopically PVL rats demonstrated granular appearance of the liver. Microscopic evaluation revealed the fibrous scars separating islands of hepatocytes, many of which contained fatty vacuoles of varying size.
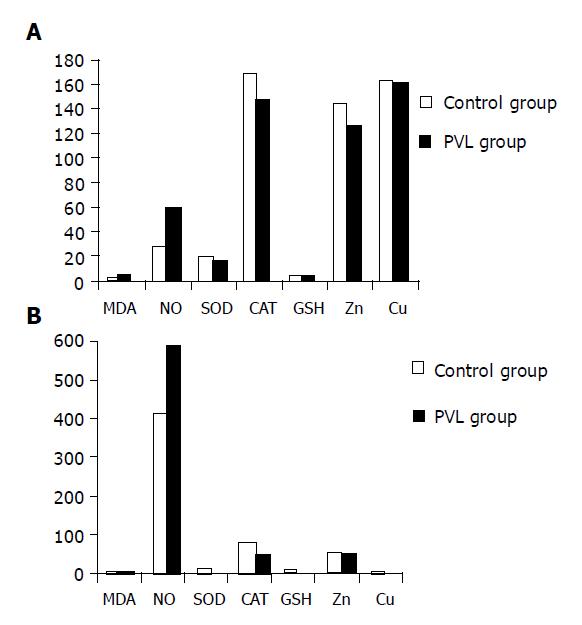
Values of the analyzed parameters and the statistical significances in the groups are shown in Table 1. The results of our study are summarized schematically in Figure 1.
| Control group (n = 10) | PVL group(n = 15) | |
| Portal pressure (cm saline) | 10.3±0.8 | 18.4±1.7b |
| MDA serum (mmol/L) | 2.7±0.25 | 4.89±0.64b |
| MDA tissue (nmol/mg protein) | 0.36±0.04 | 0.46±0.11a |
| NO serum (mmol/L) | 28.3±4.62 | 61.35±11.86b |
| NO tissue (nmol/mg protein) | 408.5±44.83 | 5812±6.88b |
| SOD serum (U/mL) | 20.8±1.81 | 15.15±1.27b |
| SOD tissue (U/mg protein) | 7.76±0.63 | 5.37±0.64b |
| CAT serum (U/mL) | 170.4±9.87 | 1471±0.25b |
| CAT tissue (U/mg protein) | 74.1±7.87 | 36.5±5.61b |
| GSH erythrocyte (mg/g Hb) | 5.24±0.51 | 3.74±0.49b |
| GSH tissue (nmol/mg protein) | 4.33±0.33 | 3.53±0.29b |
| Zn serum (mg/mL) | 145.3±16.5 | 126.2±38.4b |
| Zn tissue (mg/g) | 48.43±6.29 | 45.9±2.87 |
| Cu serum (mg/mL) | 164.5±41.4 | 161.8±38.2 |
| Cu tissue (mg/g) | 3.32±0.87 | 3.44±0.54 |
Lipid peroxidation levels as assessed by MDA in plasma and tissue increased (P<0.001, and P<0.05, respectively) in PVL group as compared to the sham-operated control group. Similarly, plasma and tissue NO levels were significantly higher in the experimental group (P<0.001).
Each of plasma and tissue antioxidant components (SOD, CAT, and GSH) was significantly lower in PVL group than in control group (P<0.001). There was no significant difference in plasma Cu and tissue Cu and Zn concentrations between the groups (P>0.05). However, plasma Zn level was significantly lower in PVL group (P<0.001).
In recent years, the role of reactive oxygen species and NO in the development of cirrhosis and portal hypertension has been extensively studied[1-3,13-15]. A role for oxidative stress in the development of hyperdynamic circulation in portal hypertension has been proposed[1,2]. On the other hand, evaluation of the limited available data indicates that it is premature to conclude that oxidative stress plays a primary role in the pathogenesis of cirrhosis.
In particular, the effect of NO on vascular function in the systemic circulation and the hepatic microcirculation has received the greatest attention. On the one hand, increased NO synthesis is responsible for the development of hyperdynamic circulation in cirrhosis, while decreased production of NO within the hepatic microcirculation may be important in the development of parenchymal tissue damage and the onset of portal hypertension[16,17]. However, many areas are still controversial.
The role of trace elements in the pathogenesis of cirrhosis has also been described recently[4,18,19]. Zinc is an essential trace element and functions as an antioxidant. Regarding all these oxidant and antioxidant parameters, the present study was undertaken to evaluate the oxidative stress status in cirrhosis from all perspectives.
We investigated the oxidative-stress-related changes in cirrhotic rats. The results showed a significant increase in the level of plasma and tissue MDA, which is known as a lipid peroxidation parameter. On the other hand, rats exhibited reduced plasma and tissue levels of SOD, GSH, and CAT, which are known as antioxidant defense enzymes. Our results support the previous studies[1,20,21]. These data may show that the depletion of antioxidant defense system can be a compensatory mechanism against the increase in the oxidative stress due to the pathological changes seen in cirrhosis.
Loguercio et al[22], also showed that GSH and its related enzymes are one of the protective mechanisms against the oxidative damage, both in circulation and in various tissues, including liver. They showed that patients with liver cirrhosis frequently suffer from hepatopathy and present low circulating levels of GSH. Similarly, our findings indicate that GSH-related cellular defensive mechanisms are depressed in experimental cirrhosis and therefore susceptibility to oxidative damage may increase.
In liver cirrhosis, an increase in hepatic resistance is the initial phenomenon leading to portal hypertension. This is primarily due to the structural distortion of intrahepatic microcirculation caused by cirrhosis. However, similar to other vascular conditions, architectural changes in the liver are suggested to be associated with deficient nitric oxide (NO) production, which results in an increased vascular tone with a further increase in hepatic resistance and portal pressure[16,24]. However, our results showed that NO production increased both in serum and in liver tissue. In our opinion, the histopathological changes in the liver are more dependent upon oxidative stress, and the reactive NO rise in liver tissue can be explained by its antioxidant protective defense mechanism.
The hyperdynamic circulation of cirrhosis and portal hypertension are due to the vasodilatory effects of nitric oxide[3,23]. Increased release of nitric oxide (NO) plays a role in the pathogenesis of vasodilatation and vascular hypocontractility, leading to portal hypertension[25-27]. In our study, the rise in NO concentration in serum of PVL rats was significant, and this finding supports the NO-mediated portal vasodilation hypothesis.
Trace metals have also been shown to play an important role in the pathogenesis of liver cirrhosis and the development of portal hypertension[4]. Zinc is an essential trace element and functions as an antioxidant. Low zinc concentrations have been reported in patients with cirrhosis of the liver, particularly those with hepatic encephalopathy[18]. Patients with fulminant and subacute hepatic failure have low serum zinc levels[28,29]. Scholmerich et al[19], showed that patients with surgical portosystemic shunt have significantly lower levels of zinc, vitamin A and retinol-binding protein than controls and patients with cirrhosis without shunt. Patients with portal hypertension who are considered to have spontaneous shunting also have lower levels than those without this symptom. Similarly, the present study showed that Zn concentration significantly decreased in serum of the experimental group. Since Zn plays a role as an antioxidant agent, the data may prove the change in rats with portal hypertension.
Experimental and clinical studies suggested that xenobiotic hepatotoxicity with variable depletion of antioxidants can be avoided or ameliorated by administration of an unusually high dosage of zinc or by a combination of antioxidants above normal daily requirements[29]. Therefore, reassessment of optimal prophylactic and therapeutic nutritional requirements of antioxidants (particularly zinc) to protect humans against xenobiotic-induced oxidative stress is advocated.
It has been shown that hepatic copper overload may contribute to the development of hepatocellular carcinoma in HCV-positive patients with chronic hepatitis or cirrhosis[18,28]. However, our results did not support this hypothesis as we could not find any remarkable change in both serum and tissue Cu concentrations. In our opinion, Cu may have a local effect on liver cirrhosis at an advanced stage. On the other hand, we are unable to explain the mechanism with the current available data.
In conclusion, these findings show that excessive formation of NO may be responsible, at least in part, for the hemodynamic derangements in cirrhosis. Although oxidative stress and trace metals may not participate in the initiation of hyperdynamic circulation in cirrhosis, they may play a primary role in the pathogenesis of cirrhosis and contribute to the maintenance of hyperdynamic circulation observed in cirrhotic rats with ascites.
| 1. | Huang YT, Hsu YC, Chen CJ, Liu CT, Wei YH. Oxidative-stress-related changes in the livers of bile-duct-ligated rats. J Biomed Sci. 2003;10:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Bomzon A, Ljubuncic P. Oxidative stress and vascular smooth muscle cell function in liver disease. Pharmacol Ther. 2001;89:295-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wang H, Chen XP, Qiu FZ. Salviae miltiorrhizae ameliorates cirrhosis and portal hypertension by inhibiting nitric oxide in cirrhotic rats. Hepatobiliary Pancreat Dis Int. 2003;2:391-396. [PubMed] |
| 4. | Chetri K, Choudhuri G. Role of trace elements in hepatic encephalopathy: zinc and manganese. Indian J Gastroenterol. 2003;22 Suppl 2:S28-S30. [PubMed] |
| 5. | Hiiemae K, Rozmiarek H, Williams JF, LeBeau JE, Ross M. Report of a panel discussion on how to run an effective Animal Care and Use Committee. Lab Anim Sci. 1987;37 Spec No:39-44. [PubMed] |
| 6. | Hsieh JS, Huang TJ. The effect of portal hypertension on gastric epithelial proliferation in rats. Eur Surg Res. 1993;25:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Angel MF, Ramasastry SS, Swartz WM, Narayanan K, Kuhns DB, Basford RE, Futrell JW. The critical relationship between free radicals and degrees of ischemia: evidence for tissue intolerance of marginal perfusion. Plast Reconstr Surg. 1988;81:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Lowry OH, Rosenbrough NJ, Farr AC, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 9. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9148] [Article Influence: 207.9] [Reference Citation Analysis (0)] |
| 10. | Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497-500. [PubMed] |
| 11. | Yasmineh WG, Kaur TP, Blazar BR, Theologides A. Serum catalase as marker of graft-vs-host disease in allogeneic bone marrow transplant recipients: pilot study. Clin Chem. 1995;41:1574-1580. [PubMed] |
| 12. | Beutler E, Duran O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882-888. [PubMed] |
| 13. | Evelson P, Llesuy S, Filinger E, Rodriguez RR, Lemberg A, Scorticati C, Susemihl M, Villareal I, Polo JM, Peredo H. Decreased oxidative stress in prehepatic portal hypertensive rat livers following the induction of diabetes. Clin Exp Pharmacol Physiol. 2004;31:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Huang HC, Wang SS, Chan CC, Lee FY, Chang FY, Lin HC, Hou MC, Tai CC, Lai IN, Lee SD. Chronic inhibition of nitric oxide increases the collateral vascular responsiveness to vasopressin in portal hypertensive rats. J Hepatol. 2004;40:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Oztürk H, Yağmur Y, Buyukbayram H, Dokucu AI, Gurel A. Effects of the nitric oxide donor molsidomine on the early stages of liver damage in rats with bile duct ligation: a biochemical and immunohistochemical approach. Eur Surg Res. 2002;34:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | González-Abraldes J, García-Pagán JC, Bosch J. Nitric oxide and portal hypertension. Metab Brain Dis. 2002;17:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Bhimani EK, Serracino-Inglott F, Sarela AI, Batten JJ, Mathie RT. Hepatic and mesenteric nitric oxide synthase expression in a rat model of CCl(4)-induced cirrhosis. J Surg Res. 2003;113:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Göksu N. Hepatic and serum levels of zinc, copper, and magnesium in childhood cirrhosis. J Pediatr Gastroenterol Nutr. 1986;5:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Schölmerich J, Becher MS, Köttgen E, Rauch N, Häussinger D, Löhle E, Vuilleumier JP, Gerok W. The influence of portosystemic shunting on zinc and vitamin A metabolism in liver cirrhosis. Hepatogastroenterology. 1983;30:143-147. [PubMed] |
| 20. | Kaur S, Kaur U, Tandon C, Dhawan V, Ganguly NK, Majumdar S. Gastropathy and defense mechanisms in common bile duct ligated portal hypertensive rats. Mol Cell Biochem. 2000;203:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Naito Y, Yoshikawa T, Boku Y, Fujii T, Masui Y, Tanaka Y, Fujita N, Yoshida N, Kondo M. Protective role of intracellular glutathione against nitric oxide-induced necrosis in rat gastric mucosal cells. Aliment Pharmacol Ther. 2000;14 Suppl 1:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Loguercio C, Taranto D, Vitale LM, Beneduce F, Del Vecchio Blanco C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free Radic Biol Med. 1996;20:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Shams V, Erkan T, Gümüstas MK, Cullu F, Kutlu T, Kaya H, Aydin S, Tümay G. The role of nitric oxide in pediatric patients with portal hypertension. J Trop Pediatr. 2003;49:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver. 2001;21:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Yang W, Benjamin IS, Moore K, Portmann B, Alexander B. The action of nitric oxide on hepatic haemodynamics during secondary biliary cirrhosis in the rat. Eur J Pharmacol. 2003;461:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Kiefer FN, Neysari S, Humar R, Li W, Munk VC, Battegay EJ. Hypertension and angiogenesis. Curr Pharm Des. 2003;9:1733-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Arkenau HT, Stichtenoth DO, Frölich JC, Manns MP, Böker KH. Elevated nitric oxide levels in patients with chronic liver disease and cirrhosis correlate with disease stage and parameters of hyperdynamic circulation. Z Gastroenterol. 2002;40:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ebara M, Fukuda H, Hatano R, Yoshikawa M, Sugiura N, Saisho H, Kondo F, Yukawa M. Metal contents in the liver of patients with chronic liver disease caused by hepatitis C virus. Reference to hepatocellular carcinoma. Oncology. 2003;65:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp Mol Pathol. 2003;75:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |